Abstract
Cavernous hemangiomas rarely involve the female genital tract. It is difficult to identify vascular malformations when these lesions are concealed in the vagina or deep vulva area. We present a rare case of vaginal cavernous hemangioma in a 30-year-old primiparous woman with an early severe postpartum hemorrhage (PPH) and delayed continuous bleeding from the episiotomy site. She was treated successfully with transarterial embolization of the left vaginal artery. To our knowledge, this is the first reported case of PPH caused by rupture of a vaginal hemangioma during vaginal delivery in English literature.
Cavernous hemangioma is a benign vascular tumor that develops with wide distribution in internal organs or on the skin. The incidence of cavernous hemangioma is different according to its original location. They are commonly located in the head and neck area; however, female genital tract hemangiomas are relatively rare. They are usually asymptomatic small lesions that are observed incidentally during routine gynecological examinations [1]. Hemangiomas can undergo spontaneous resolution. The treatment of hemangiomas may include intra-lesional glucocorticoid therapy, the use of interferon-α, laser therapy, and surgical excision. Complications, such as rapid growth and bleeding, have been reported in genital tract hemangiomas during pregnancy [2].
A 30-year-old primiparous woman suffered from severe hemorrhage immediately after episiotomy and complained about continuous vaginal bleeding from the episiotomy site after vaginal delivery. When an undiagnosed hemangioma is located at the area where episiotomy is planned, unpredictable massive hemorrhage can occur. We describe a case of serious uncontrollable hemorrhage due to the rupture of an undiagnosed vaginal cavernous hemangioma, caused by an episiotomy incision during vaginal delivery. We also describe successful arterial embolization to treat intractable bleeding from vaginal hemangioma.
A 30-year-old primiparous woman was transferred to our obstetric unit for delayed postpartum hemorrhage (PPH). She underwent vaginal delivery 3 weeks ago at a local obstetric clinic and gave birth to a healthy baby weighing 3,500 g at 40 weeks of gestation. She had no past or familial, medical, or surgical history of the condition. Medical records from the local clinic described massive bleeding from the episiotomy site, and her blood pressure was 80/50 mmHg. Five units of packed red blood cell were transfused. The physicians experienced difficulty in controlling the bleeding from the episiotomy site for 3 hours. Also, multiple sutures were placed at the episiotomy wound but oozing continued at every stitch. The episiotomy was resutured 3 times. The vaginal wall was compressed with Vaseline roll gauze. Although she no longer had severe bleeding after these procedures, vaginal spotting continued if vaginal wall compression was not applied. Physical examination revealed that the posterior and left sides of the vaginal wall were bulgy and bluish, and a small amount of bleeding continued from the median episiotomy suture site 3 cm above the hymen. There was no laceration on the cervix and vagina except for the episiotomy wound. The uterus was firmly palpated, and its cavity was clean on transabdominal ultrasonography. Her vital signs were stable. Her hemoglobin level was 10.5 g/dL. The other laboratory results, including the tests for liver function, electrolytes, urinalysis, C-reactive protein, and coagulation profiles, were normal.
She received fluid therapy with intravenous hemostatic agents, and the vaginal wall was compressed again with a Vaseline gauze. On hospital day 3, vaginal bleeding continued, and we resutured and electrocauterized the episiotomy and applied various local hemostatic agents (Tachosil®, Takeda, Osaka, Japan; Cutanplast Sponge Standard®, Mascia Brunelli, Milano, Italy) on the bleeding point. The vaginal bleeding decreased significantly after the procedure, and the patient was discharged with antibiotics on postoperative day 3.
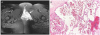
She revisited our obstetric unit for evaluation of the episiotomy condition 2 weeks later. Contrary to our expectation, she still had vaginal spotting on a coin-sized elevated raw surface of the posterior vaginal wall. She recalled she had clitoromegaly and was diagnosed with clitoral hemangioma treated by clitoral wedge resection 12 years ago. During the prenatal period, she unintentionally did not mention the hemangioma to her doctor. We supposed that the bluish bulged vaginal lesion was related to the previous clitoral hemangioma. We recommended pelvic magnetic resonance imaging (MRI) for detection of a concealed hemangioma in the vagina and performed deep punch biopsy in the upper episiotomy site of the vaginal wall. Acute bleeding from the biopsy site was controlled by compression with a Vaseline gauze and the patient was promptly referred to a radiologist for vascular intervention as planned. Pelvic MRI revealed a 7.8-cm hemangioma with a long axis in the left vaginal area (Fig. 1A). Pathologically, cavernous hemangioma was confirmed by the large dilated vessels lined by flattened endothelium. Many blood vessels were filled with red blood cells agglomerated together (Fig. 1B). After superselection of the left vaginal artery, transarterial embolization (TAE) with Gelfoam was performed. (Fig. 2). Vaginal bleeding finally stopped on postembolization day 3, and she was discharged without acute complications. After 1 month, she was doing well, and vaginal bleeding had not recurred.
A dilated vascular structure would be observed in the cases of varix, arteriovenous malformation, hemangioma, vascular tumor, and lymphatic malformation. The gross findings of those vascular lesions look similar, but their prognoses and therapeutic methods are different from each other. Therefore, a differential diagnosis is very important to manage those vascular diseases. Hemangiomas are proliferative hamartomas of the vascular endothelium that can grow on any part of the human body at any time in life. However, the incidence of female genital tract hemangioma is rare, especially during pregnancy [234]. Cavernous hemangiomas are collections of dilated vessels in the deep dermis and/or subcutaneous tissue. Their lesions are cystic, firm, or compressible, and the overlying surface may appear normal or bluish in color. Simple tissue biopsy for the purpose of diagnosis can cause massive bleeding from abundant vessels. Before the excisional biopsy, pelvic MRI, angiography, or color Doppler must be considered to diagnose and determine the extent of the disease [56].
Several cases have been reported regarding the relationship between hemangioma and pregnancy. Pregnancy sometimes induces the enlargement of tumors and rapid development of symptoms from the usual asymptomatic lesions. During pregnancy, the enlargement of a hepatic hemangioma and the onset of symptoms in patients with previously asymptomatic vertebral hemangioma have been reported [78]. The mechanism of hemangioma growth during pregnancy remains unclear. Cavernous hemangioma can be affected by sex hormones, especially estrogen, like other hemangiomas [9]. The alterations in the female sex hormonal level during pregnancy may be related to the increasing size of a hemangioma. New symptoms develop due to the enlargement of a hemangioma. Like hepatic hemangiomas, vaginal or cervical hemangiomas can increase and become symptomatic during pregnancy. In a case of diffuse cavernous hemangioma of uterus, which was not detected antenatally, hysterectomy was performed for uncontrollable bleeding during cesarean section [10]. In particular, female genital tract hemangiomas could complicate normal vaginal delivery due to the obstruction of the birth canal and massive hemorrhage by tumor rupture. When a pregnant woman has a history of genital hemangioma, thorough physical examination and imaging study before delivery should be conducted to evaluate any changes in the present hemangioma and to detect other undiagnosed hemangiomas. In this case, because the vaginal hemangioma was located in a deep area and extended into the upper perineum, we believed that her vaginal hemangioma had already been concurrent with the past clitoral hemangioma before she was pregnant.
The successful vaginal delivery after the careful excision of vaginal hemangioma before giving birth has been reported [2]. If the vaginal hemangioma is small and located on a superficial area, vaginal delivery after excision may be possible. However, cavernous hemangiomas are usually located in deep tissues and it is difficult to excise them completely without significant blood loss.
Episiotomy and vaginal lacerations are the common cause of PPH. They are occasionally large enough to require extensive repair for hemostasis. If the patient has no coagulation disorder, multiple hemostatic sutures are efficient to control bleeding from the laceration wound. In this case, PPH occurred when the pre-existing vaginal hemangioma was injured by episiotomy during vaginal delivery. It is hard to be considered that hemangioma is a cause of PPH in general obstetric condition. Without knowing the past history of genital tract hemangioma, an erroneous decision about the delivery mode and episiotomy was taken. Even after delivery, we were unaware of the exact cause of intractable continuous bleeding from episiotomy site till a pelvic MRI was performed after knowledge about the medical history of clitoral hemangioma. Severe and continuous bleeding from ruptured hemangioma could not be controlled by routine episiotomy repair techniques. Because her left vaginal hemangioma was extensive from the vulva to the mons pubis longitudinally and from the left lateral pelvic wall to the median vaginal area transversely, we assumed that the excision of hemangioma may result in excessive amount of blood loss and complete resection was very difficult. Individualized treatment plan should be made in consideration of each patient's circumstances like pregnancy status, the extent of the disease, and the location of the lesion. TAE has been shown to be effective in the management of surgically unresectable hepatocellular carcinomas [11] and vaginal hemangioma during the postpartum period [12]. The patient was recommended TAE therapy and the left vaginal arterial embolization was a successful treatment for intractable bleeding from the injured hemangioma. After TAE, the decrease in the size of the hemangioma may be associated with the histopathological degeneration caused by coagulative necrosis within the tumor [12].
In summary, genital tract hemangiomas can be a cause of severe PPH, and patients diagnosed with this disorder should undergo thorough physical examinations and imaging studies to identify the problematic hemangiomas that induce serious hemorrhage during vaginal delivery. Cesarean section may be preferred to vaginal delivery when cavernous hemangioma is large and deep in location. TAE is an effective therapy for PPH caused by the rupture of genital tract hemangioma.
Figures and Tables
Fig. 1
(A) Pelvic magnetic resonance imaging finding of vaginal hemangioma. A high signal globular lesion in the left lower vaginal wall is seen on T2 fat suppression image (arrow). It extends from the vulva to the mons pubis longitudinally, and from the left lateral pelvic wall to the median vaginal wall transversely. (B) Microscopic finding of the vaginal wall. A medium power field view of the vaginal wall reveals aggregates of large, thin-walled vessels lined by flattened endothelial cells and containing red blood cells (H&E, ×100). H&E, hematoxylin and eosin.
References
1. Kondi-Pafiti A, Kairi-Vassilatou E, Spanidou-Carvouni H, Kontogianni K, Dimopoulou K, Goula K. Vascular tumors of the female genital tract: a clinicopathological study of nine cases. Eur J Gynaecol Oncol. 2003; 24:48–50.
2. Celik F, Arioz DT, Köken GN, Yilmazer M. Very rare cause of vaginal mass in pregnancy: cavernous hemangioma. J Obstet Gynaecol Res. 2012; 38:889–891.
3. Gupta R, Singh S, Nigam S, Khurana N. Benign vascular tumors of female genital tract. Int J Gynecol Cancer. 2006; 16:1195–1200.
4. Rezvani FF. Vaginal cavernous hemangioma in pregnancy. Obstet Gynecol. 1997; 89:824–825.
5. Cebesoy FB, Kutlar I, Aydin A. A rare mass formation of the vulva: giant cavernous hemangioma. J Low Genit Tract Dis. 2008; 12:35–37.
6. Wang S, Lang JH, Zhou HM. Venous malformations of the female lower genital tract. Eur J Obstet Gynecol Reprod Biol. 2009; 145:205–208.
7. Saegusa T, Ito K, Oba N, Matsuda M, Kojima K, Tohyama K, et al. Enlargement of multiple cavernous hemangioma of the liver in association with pregnancy. Intern Med. 1995; 34:207–211.
8. Chi JH, Manley GT, Chou D. Pregnancy-related vertebral hemangioma. Case report, review of the literature, and management algorithm. Neurosurg Focus. 2005; 19:E7.
9. Conter RL, Longmire WP Jr. Recurrent hepatic hemangiomas. Possible association with estrogen therapy. Ann Surg. 1988; 207:115–119.
10. Virk RK, Zhong J, Lu D. Diffuse cavernous hemangioma of the uterus in a pregnant woman: report of a rare case and review of literature. Arch Gynecol Obstet. 2009; 279:603–605.
11. Taourel P, Dauzat M, Lafortune M, Pradel J, Rossi M, Bruel JM. Hemodynamic changes after transcatheter arterial embolization of hepatocellular carcinomas. Radiology. 1994; 191:189–192.
12. Emoto M, Tamura R, Izumi H, Sukimoto S, Kawarabayashi T, Shirakawa K. Sonodynamic changes after transcatheter arterial embolization in a vaginal hemangioma: case report. Ultrasound Obstet Gynecol. 1997; 10:66–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download