Abstract
Ovarian tumors are relatively uncommon in paediatric age group and mostly occur in the period after menarche and are extremely rare prior to menarche. However, in children and adolescent, the epithelial ovarian tumors account approximately 10% to 28% of all ovarian tumors. In the present case, the patient was presented with abdominal pain for 1 day and no mass was felt on physical examination. Contrast-enhance computed tomography abdomen and pelvis showed a left ovarian cystic mass measuring 7.3×5.1 cm in size and unilateral oophorectomy was done. Tumor shows multiloculated cysts of varying sizes filled with mucinous fluid. Cysts were lined by tall columnar epithelial cells and show predominantly periodic acid-Schiff positive neutral mucin favouring benign nature of lesion. We present this case due to its uncommon age of presentation and the patient was premenarchal girl.
Ovarian tumors are relatively uncommon in paediatric age group and accounts approximately less than 2% of all childhood tumors and 6% of ovarian tumors in all ages [1]. In the premenarchal stage, the ovarian mass range from nonneoplastic lesion such as benign functional cysts to malignant tumors mostly germ cell in origin [12]. The ovarian tumors commonly seen in the second decade of childhood usually present with nonspecific clinical signs and symptom [12]. Therefore, it is difficult to make correct diagnosis preoperatively [12]. Early treatment is required to preserve the fertility and patient's life [12]. The epithelial ovarian tumors account approximately 8% to 10% of all ovarian tumors [12]. However, in children and adolescent, the epithelial ovarian tumors account approximately 10% to 28% of all ovarian tumors [12]. The epithelial ovarian tumors are histologically classified as mucinous or serous and cystadenoma is the most common benign epithelial tumor of which 75% are serous and 25% are mucinous [12]. We present a case of benign mucinous cystadenoma (MCA) in a premenarchal 12-year-old girl due to its rarity as the only less than 25 cases of MCA in premenarchal girls are described in the literature searched using the MEDLINE database through PubMed.
A 12-year-old girl presented in gynaecology outpatient department with complaint of abdominal pain for 1 day. Patient was in tanner stage 2 breast development and 2 pubic hair growth, however menarche was not attained. On physical examination, abdomen was soft, nontender, and no mass was felt. The patient's routine blood analysis, liver function test, and renal function test were within normal limits: hemoglobin, 12.9 g/dL (11.5–15.5 g/dL); total leukocytes count, 9.5×103/mm3; total bilirubin, 0.7 mg/dL (0.1–1.2 mg/dL); alanine transaminase, 22 units/L (4–36 U/L); aspartate aminotransferase, 20 units/L (8–33 U/L); urea, 16 mg/dL (15–40 mg/dL); and creatinine, 0.65 mg/dL (0.6–1.2 mg/dL). The levels of follicle-stimulating hormone and luteinizing hormone were 6.5 mIU/mL (<0.1–4.3 mIU/mL) and 3.3 mIU/mL (<0.1–5.0 mIU/mL), respectively. The tumor markers were cancer antigen 125 (CA-125), 10.2 U/mL (0–35 U/mL); human chorionic gonadotropin, 1.3 IU/L (0–5 IU/L); carcinoembryonic antigen, 1.6 µg/L (0–3 ng/mL).
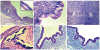
The contrast-enhance computed tomography (CECT) of the abdomen and pelvis showed a well-defined, capsulated, multiseptate left ovarian cystic mass measuring 7.3×5.1 cm in size and was reported as benign cystadenoma (Fig. 1A). Exploratory laparotomy was performed and noted that the cystic mass was originated from the left ovary. No signs of metastasis were observed in the pelvis, on abdominal wall, diaphragm surface, and peritoneum. The right-side ovary appeared normal. Left oophorectomy was performed and specimen was sent for histopathology examination. On gross examination, oophorectomy specimen was well encapsulated, greyish white cystic, ovarian mass with smooth and glistening surface (Fig. 1B). Cut surface showed multiloculated cysts of varying sizes filled with mucinous fluid (Fig. 1C). No solid areas were seen. Microscopic examination showed multiple cysts lined by tall columnar epithelial cells with basally located round to oval nuclei and abundant pale cytoplasm. Focal area of calcification was also seen (Fig. 2A-2D). No invasion, atypical mitosis or necrosis was seen. On histocytochemistry by periodic acid-Schiff (PAS) with or without diastase and alcian blue-PAS (ABPAS) showed only PAS positivity in the lining epithelial cells (Fig. 2E and 2F). Based on histopathological examination, a final diagnosis of MCA of left ovary was made. Post-operative period and 6 months follow-up was uneventful. The patient was advised to continue regular follow-up.
Epithelial ovarian tumors are rare in children and adolescents mostly occur in the period after menarche and are extremely rare prior to menarche [23]. During ovulation process, the repeated repair and disruption of surface epithelium with subsequent higher risk of spontaneous mutations may be the cause of epithelial ovarian tumors [3]. These tumors are also stimulated by hormones as the incidence increases after menarche [13]. In premenarchal girls, molecular events are different from those of adults which may play role in pathogenesis of MCA [4]. MCA may present with nonspecific symptoms such as acute or chronic abdominal pain, abdominal distension, ovarian torsion and symptoms due to pressure on urinary bladder, rectum and vessels [135].
Some tumor markers are used for differential diagnosis preoperatively and may be used to monitor complete resection of tumor as well as relapse postoperatively [1]. CA-125, a high-molecular-weight glycoprotein has possible use to identifying ovarian epithelial tumors as it is specific for epithelial differentiation and related to tumor volume [1]. In MCA, tumor marker CA-125 and carcinoembryonic antigen level may be normal or elevated [12345]. However, α-fetoprotein and β-human chorionic gonadotropin levels are within normal limits [12345].
The ultrasound, computed tomography, and magnetic resonance imaging are important techniques for evaluating the location, size, and metastasis of the tumor [1]. For the palpable abdominal masses, ultrasound is usually the first choice of radiological investigation [1]. MCA are usually unilateral, large cystic and <10% cases are bilateral [15]. Approximately three-fourths cases are multilocular and one-fourth cases are unilocular [15]. Cut surface shows multiple smooth walled cysts of varying size filled with mucinous fluid secreted by lining epithelium [5]. In all mucinous ovarian neoplasms, 77% to 87% are benign [45]. Microscopically these cysts are lined by endocervical or intestinal mucinous epithelium [45]. Many times, an area of focal calcification, a common indicator of benignity is also present [4]. Fan observed that 3 out of 4 cases of premenarchal MCA had some degree of serous epithelium lining [4].
In the present case, the patient was premenarchal presented with abdominal pain with normal serum assay for tumor markers and CECT revealed a well-defined, capsulated, multiseptate left ovarian cystic tumor. Grossly tumor was unilateral, large, multiloculated cystic and cysts were filled with mucinous fluid. Microscopically tumor lining epithelium was predominantly endocervical type and showed focal area of calcification.
In MCA, different proportion of neutral and acidic mucin are seen extracellularly and intracellularly demonstrated by PAS and alcian blue stain [6]. Sialomucin is the predominant acidic mucin in MCA if present [6]. As MCA progresses to borderline MCA to cystadenocarcinoma the proportion of neutral mucin decreases and acidic mucin predominantly sulfated mucin increases [6]. In the present case, lining epithelium showed only diastase resistant PAS positive and alcian blue negative intracytoplasmic mucin which support the benignity of the tumor.
The differential diagnosis ovarian masses in the premenarchal period include benign and malignant tumors, torsion, cyst, haematological malignancy involving the ovary, and metastatic diseases. In the present case, CECT clearly identify the origin of mass and easily diagnosed on histopathology. The cystectomy or ipsilateral oophorectomy or salpingooophorectomy is the usual treatment of benign ovarian tumors [12]. Management strategies should keep in mind to cure and preserve the fertility function in premenarchal girls [12]. Unilateral oophorectomy was the therapeutic strategy in the present case. The ovarian MCA had good prognosis because malignant transformation occurs only in 5% to 10% of patients [1]. In view of possible recurrence or affects contralateral ovary follow-up once every 3 months through both radiologically and serologically during the first year and once a year thereafter. After continue follow-up of 9 months, there was no recurrence reported in the present case.
In conclusion, the diagnosis of MCA is often is delayed due to its age of presentation in this case and rarity of MCA in premenarchal girls. Management strategies should keep in mind to cure and preserve the fertility function in premenarchal girls.
Figures and Tables
Fig. 1
(A) Contrast enhance computed tomography (CECT): hypodense cystic tumor with enhancing septations. (B) Ovarian cyst with smooth external surface. (C) Cut surface: multilocular cyst.

Fig. 2
(A) Mucinous endocervical type epithelium (H&E, ×10, ×40 inset). (B) Mucinous endocervical-type epithelium with basally located nuclei (H&E, ×40). (C) Ovarian stroma with graafian follicles (H&E, ×40). (D) Mucinous epithelium with calcification (H&E, ×40). (E) Diastase resistant periodic acid-Schiff (PAS) positivity in lining epithelium (PAS-diastase, ×40). (F) PAS positive alcian blue negative in lining epithelium (Alcian blue-PAS, ×40).
References
1. Cevik M, Guldur ME. An extra-large ovarian mucinous cystadenoma in a premenarchal girl and a review of the literature. J Pediatr Adolesc Gynecol. 2013; 26:22–26.
2. Biçer S, Erkul Z, Demiryilmaz I, Peker N. A 9-kg ovarian mucinous cystadenoma in a 14-year-old premenarchal girl. Am J Case Rep. 2014; 15:326–329.
3. Virgone C, Alaggio R, Dall'Igna P, Buffa P, Tonegatti L, Ferrari A, et al. Epithelial tumors of the ovary in children and teenagers: a prospective study from the Italian TREP Project. J Pediatr Adolesc Gynecol. 2015; 28:441–446.
4. Fan R. Unique features and insights from mucinous tumors of ovary in childhood: clinicopathological study of 15 cases including four premenarchal cases. Fetal Pediatr Pathol. 2014; 33:166–175.
5. Parmentier B, Vaz E, Chabaud-Williamson M, Fasola S, Kotobi H, Coulomb-L’herminé A, et al. Mucinous cystadenoma arising 3 years after ovarian-sparing surgery for mature teratoma in a child. J Pediatr Surg. 2010; 45:E9–E12.
6. AL-Kaptan IA. Study of mucins in epithelial ovarian tumors. J Fac Med Baghdad. 2005; 47:386–391.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download