Abstract
Methods
This study conducted a retrospective chart review of 23 patients with OP and 46 patients with tubal pregnancy (TP) from October 1, 2003 to September 31, 2016 in Hanyang University Hospital.
Results
There were no significant differences in demographic and clinical characteristics between the two groups. The presence of an ectopic gestational sac and hemoperitoneum was significantly higher in the TP group (13.0% vs. 95.7%, P=0.000; 13.0% vs. 54.3%, P=0.001, respectively) in preoperative ultrasonogram. The OP group had more ruptured ectopic gestational sacs than the TP group (73.9% vs. 45.7%, P=0.039) in surgical findings.
Ectopic pregnancy is an early pregnancy complication in which a fertilized ovum is implanted outside of the uterine cavity, most commonly in the fallopian tubes. The most frequent site within the tubes is the ampullary portion, followed by the isthmus, interstitial, cornual, and fimbrial portions. In addition, it can also occur in the ovary, abdominal cavity, the uterine cervix, and at cesarean scar site. Ovarian pregnancy (OP) occurs when a fertilized ovum is implanted on the ovary which accounts for approximately 3% of total ectopic pregnancies [1].
The early diagnosis of ectopic pregnancy has become possible because of the availability of sensitive assays for human chorionic gonadotropin (hCG) and the development of transvaginal ultrasound [2]. The characteristic sonographic findings of OP include a wide echogenic ring accompanying an echolucent area within an ovary, for a definitive diagnosis, a yolk sac or embryo should be observed within the echogenic ring [3]. However, OP, ruptured corpus luteum, and tubal pregnancy (TP) are detected mainly as adnexal masses, it is not easy to perform a differential diagnosis targeting such ectopic pregnancies [4].
If OP is not diagnosed earlier, life-threatening conditions can be caused by rupture of an ectopic gestational sac and consequent hemoperitoneum resulting from the high vascularity of ovarian tissues [5]. Therefore, it is important to be aware of these rare conditions to reduce the associated morbidities.
In this study, we aimed to analyze the features allowing the early diagnosis of OP by comparing the demographic and clinical characteristics between patients with TP and OP.
This study conducted a retrospective chart review of 23 patients with OP and 46 patients with TP from October 1, 2003 to September 31, 2016 in Hanyang University Hospital. We reviewed the medical records to analyze the demographic and clinical characteristics, surgical outcomes such as gestational age at diagnosis, risk factors of ectopic pregnancy such as abortion history, pelvic inflammatory disease (PID), and tubal surgery, and the use of ovulation-inducing agents and assisted reproductive technology (ART). All patients had received an ultrasound before the surgery and serial serum concentrations of β-hCG were recorded.
Extensive hemoperitoneum was defined as fluid collection in the hepatorenal recess in preoperative ultrasound scan. Hemoperitoneum was measured by total volume of saline used for the irrigation subtracting from the total fluid volume aspirated during the operation.
The χ2 test was used to assess the differences among the 2 groups. SPSS ver. 13.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of data, with P-values <0.05 considered significant.
The clinical characteristics of the patients are summarized in Table 1. There were no significant differences between the 2 groups in terms of age, parity, body mass index, gestational age, serum β-hCG level at the surgery, risk factors of ectopic pregnancy, number of abdominal surgeries including tubal surgery, ART, and hospital stay. The most common symptom was lower abdominal pain and the next most common symptom was vaginal bleeding.
In terms of sonographic findings, there were significant differences in the ectopic gestational sac (13.3% of OP group and 95.7% of TP group) and fluid shadow in the hepatorenal recess (13.0% of the OP group and 54.3% of the TP group) between the 2 groups. Specifically, fluid collection in the hepatorenal recess is a typical sonographic finding known as extensive hemoperitoneum. However, there was no significant difference in fluid collection in the cul-de-sac between 2 groups.
Fifty-nine (19 of the OP group and 40 of the TP group) and 10 patients (4 of the OP group and 6 of the TP group) underwent laparoscopic surgery and laparotomy, respectively. There was no significant difference in the operating time of the 2 groups.
The rupture of an ectopic gestational sac was significantly more common in the OP group (73.9% in the OP group and 45.7% in the TP group) and the pelvic adhesion was significantly more common in the TP group (39.1% in the OP group and 52.3% in the TP group). However, there was no significant difference in amount of hemoperitoneum and transfusion rate in both groups (Table 2).
OP is a relatively uncommon type of ectopic pregnancy; its incidence is approximately 0.5%–3% of ectopic pregnancies, and it is known to occur with a frequency of one in 7,000–15,000 pregnancies [67]. The reported incidence of OP is growing because of an increasing awareness by physicians of the possibility of an OP and careful histologic examination of the ovarian tissues [8].
A history of tubal surgery, a prior ectopic pregnancy, PID history, and intrauterine device (IUD) use are known to be risk factors for ectopic pregnancy [91011]. Of these, PID history is a risk factor for TP, but not for OP [12], while use of an IUD is known to correlate with the incidence of OP [2]. Furthermore, the incidence of OP accompanying ovarian hyperstimulation during ART is also increasing [13].
Primary OP can be histologically classified into intrafollicular and extrafollicular OP [14]. Extrafollicular forms can be subdivided into juxtafollicular, interstitial, cortical, and superficial types. Although this classification has been widely accepted, it has no therapeutic relevance and is impractical for physicians [1516].
The natural history of OP indicates that, similar to other ectopic pregnancies, the sac usually ruptures within a certain period of time after development, but there are even cases where pregnancy proceeds up to the second or third trimester [17]. The sac most commonly ruptures and is terminated during the first trimester (91%), with 5.3% proceeding to the second trimester and 3.7% to the third trimester [18]. Uncommonly, a twin OP, a calcified fetus, or recurrent OP occur. One instance of a twin OP that developed bilaterally was reported [19]. OP does not have a deleterious effect on a woman's future reproductive capability, and it is very rare for it to recur [20].
The clinical findings of OP are very similar to those of TP or ruptured corpus luteum. In all of these, abdominal pain is the most common symptom, and all 3 show a range of symptoms, including vaginal bleeding, an adnexal mass, irregular menstruation, and shock. It has been reported that the amount of blood loss observed during laparotomy is 50–2,500 mL [21]. Approximately 50% of ovarian pregnancies that develop after ovarian stimulation can be diagnosed at an asymptomatic stage [22].
Typical sonographic finding of OP is a thick echogenic peripheral vascular ring observed around cysts in the ovary, which is similar to the sonographic findings for the corpus luteum. Therefore, a yolk sac or an embryo within an echogenic ring should be detected for a definitive diagnosis [3]. Initial serum β-hCG levels are not critical: even if an abnormally low and slow-rising serum β-hCG is of help in the early recognition of abnormal implantation, it is not specific to confirm the diagnosis of OP [23]. Diagnostic laparoscopy has emerged as a simple method for visualizing ovaries and fallopian tubes directly. One study demonstrated that its diagnostic accuracy for ectopic pregnancy is 96.3% [24].
In 1878, Spiegelberg [25] suggested the following as diagnostic criteria: 1) the fallopian tube on the affected side must be intact; 2) the gestational sac must occupy the same position as the ovary; 3) the ovary must be connected to the uterus by the utero-ovarian ligament; and 4) ovarian tissue must be located in the gestational sac wall. In 2002, Sergent et al. [20] suggested the following as new diagnostic criteria to be added to those of Spiegelberg's study [25]: 1) serum β-hCG level ≥1,000 IU/L and uterine vacuity at vaginal ultrasonography; 2) ovarian implication confirmed by surgical exploration, with bleeding, visualization of chorionic villi, or the presence of an atypical cyst on the ovary; 3) normal tubes; and 4) undetectable serum β-hCG after treatment of the ovary.
The choice of treatment for OP is surgery, and after it was reported as successful by Van Coevering and Fisher in 1988 [26], laparoscopic surgery became the standard surgical modality. Earlier, Ovarian wedge resection was a conventional method; however, as laparoscopic techniques improve, physicians started to perform laparoscopic enucleation of the OP [23].
In this study, a gestational sac was more commonly detected on preoperative ultrasonography in TP than in OP, implicating that the rupture of the gestational sac before surgery occurred more commonly in OP. It can be explained that the plentiful blood supply of the ovary itself is the major cause of these findings [2728]. However, extensive hemoperitoneum was more common in the TP group in this study. While the ovary receives blood only from the ovarian artery, the principal blood supply of the fallopian tube is through an anastomosis between the bifurcated branch originating from the upper end of the uterine artery and the ovarian artery. In addition, the venous plexus system of the tube accompanies the arterial distribution and capillary networks that are found in the subserosal, muscularis, and mucosal layers [29]. Therefore, the possibility of extensive hemoperitoneum may be increased when an ectopic gestational sac is ruptured during a TP. Another possible cause is that the median age of patients with OP was higher than that of patients with TP, although this difference was not significant because ovarian blood flow decreases with increasing women's age. Moreover, because the type of OP seen in this study was superficial and easily ruptured, it was diagnosed on average 6 days earlier than TP; this could result in a less severe hemoperitoneum. Although not statistically significant, the mean gestational age between the 2 groups showed difference (6.4 vs. 7.3 weeks). This result can suggest the tendency of the OP to rupture earlier than the TP. Further investigation in subsequent more highly powered studies will be necessary. Our findings that rupture of the ectopic gestational sac occurred more commonly in the OP group than in the TP group and that the amount of hemoperitoneum in OP was low are clinical manifestations that distinguish OP from TP and are therefore significant.
In conclusion, because the clinical manifestations of OP and TP are not clearly distinguishable, it is difficult to make a preoperative diagnosis. However, this study showed that rupture of an ectopic sac occurred more often at an early gestational age and the quantity of hemoperitoneum was smaller in the OP than in the TP group. For the patients in whom a gestational sac is not detected in the uterus or the fallopian tubes, it is important to be aware of the possibility of OP and rupture of an ovarian gestational sac to promote early diagnosis and surgical intervention.
Figures and Tables
Fig. 1
(A) Huge hematoma in the pelvic cavity. (B) A large amount of free fluid in the hepatorenal recess. (C) Left ovarian pregnancy (white arrow).
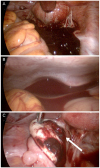
Fig. 2
(A) Small hematoma in the cul-de-sac. (B) No hematoma in the hepatorenal recess. (C) Right ovarian pregnancy (white dotted arrow).
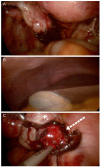
Table 1
Clinical characteristics of the patients (n=69)

Values are presented as median (range) or number (%).
OP, ovarian pregnancy; TP, tubal pregnancy; BMI, body mass index; hCG, human chorionic gonadotropin; PID, pelvic inflammatory disease; ART, assisted reproductive technology; COH, controlled ovarian hyperstimulation; IUI, intrauterine insemination; IVF, in vitro fertilization.
a)Significance at P<0.05.
Table 2
Surgical outcomes

References
1. Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002; 17:3224–3230.
2. Raziel A, Schachter M, Mordechai E, Friedler S, Panski M, Ron-El R. Ovarian pregnancy-a 12-year experience of 19 cases in one institution. Eur J Obstet Gynecol Reprod Biol. 2004; 114:92–96.
3. Baltarowich OH, Scoutt LM. Ectopic pregnancy. In : Norton ME, Scoutt LM, Feldstein VA, editors. Callen's ultrasonography in obstetrics and gynecology. 6th ed. Philadelphia (PA): Elsevier;2016. p. 966–1000.
4. Comstock C, Huston K, Lee W. The ultrasonographic appearance of ovarian ectopic pregnancies. Obstet Gynecol. 2005; 105:42–45.
5. Zhu Q, Li C, Zhao WH, Yuan JJ, Yan MX, Qin GJ, et al. Risk factors and clinical features of ovarian pregnancy: a case-control study. BMJ Open. 2014; 4:e006447.
6. Saráchaga M, Vasconcelos RM, Gantús V. Ovarian pregnancy. Presentation of a case and review of the literature. Ginecol Obstet Mex. 1992; 60:152–154.
7. Selo-Ojeme DO, GoodFellow CF. Simultaneous intrauterine and ovarian pregnancy following treatment with clomiphene citrate. Arch Gynecol Obstet. 2002; 266:232–234.
8. Mall AK. Ovarian pregnancy. A case report emphasizing the need for careful histologic review. J Reprod Med. 1996; 41:453–454.
9. Voedisch AJ, Frederick CE, Nicosia AF, Stovall TG. Early pregnancy loss and ectopic pregnancy. In : Berek JS, Novak E, editors. Berek & Novak's gynecology. 15th ed. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins;2012. p. 620.
10. Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril. 1996; 65:1093–1099.
11. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ. 2005; 173:905–912.
12. Voedisch AJ, Frederick CE, Nicosia AF, Stovall TG. Early pregnancy loss and ectopic pregnancy. In : Berek JS, Novak E, editors. Berek & Novak's gynecology. 15th ed. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins;2012. p. 643.
13. Bontis J, Grimbizis G, Tarlatzis BC, Miliaras D, Bili H. Intrafollicular ovarian pregnancy after ovulation induction/intrauterine insemination: pathophysiological aspects and diagnostic problems. Hum Reprod. 1997; 12:376–378.
14. Tan KK, Yeo OH. Primary ovarian pregnancy. Am J Obstet Gynecol. 1968; 100:240–249.
15. Boronow RC, McElin TW, West RH, Buckingham JC. Ovarian pregnancy: report of four cases and a thirteen-year survey of the English literature. Am J Obstet Gynecol. 1965; 91:1095–1106.
16. Einenkel J, Baier D, Horn LC, Alexander H. Laparoscopic therapy of an intact primary ovarian pregnancy with ovarian hyperstimulation syndrome: case report. Hum Reprod. 2000; 15:2037–2040.
17. Stanley JR, Harris AA, Gilbert CF, Dellinger EH. Magnetic resonance imaging in evaluation of a second-trimester ovarian twin pregnancy. Obstet Gynecol. 1994; 84:648–652.
18. Das S, Kalyani R, Lakshmi V, Harendra Kumar ML. Ovarian pregnancy. Indian J Pathol Microbiol. 2008; 51:37–38.
19. Köhler B, Geissler U. Bilateral ovarian pregnancy--a rare case of extrauterine pregnancy. Zentralbl Gynakol. 1983; 105:1002–1005.
20. Sergent F, Mauger-Tinlot F, Gravier A, Verspyck E, Marpeau L. Ovarian pregnancies: revaluation of diagnostic criteria. J Gynecol Obstet Biol Reprod (Paris). 2002; 31:741–746.
21. Melilli GA, Avantario C, Farnelli C, Papeo R, Savona A. Combined intrauterine and ovarian pregnancy after in vitro fertilization and embryo transfer: a case report. Clin Exp Obstet Gynecol. 2001; 28:100–101.
22. Marcus SF, Brinsden PR. Primary ovarian pregnancy after in vitro fertilization and embryo transfer: report of seven cases. Fertil Steril. 1993; 60:167–169.
23. Aydin T, Yucel B, Aksoy H, Ekemen S. Successful laparoscopic management of a rare complication after embryo transfer: ovarian pregnancy. A case report and up-to-date literature review. Wideochir Inne Tech Malo Inwazyjne. 2016; 10:574–579.
24. Grimes HG, Nosal RA, Gallagher JC. Ovarian pregnancy: a series of 24 cases. Obstet Gynecol. 1983; 61:174–180.
25. Spiegelberg O. Zur casuistik der ovarial schwangerschaft. Arch Gynaekol. 1878; 13:73–79.
26. Van Coevering RJ, Fisher JE. Laparoscopic management of ovarian pregnancy: a case report. J Reprod Med. 1988; 33:774–776.
27. Alalade A, Mayers K, Abdulrahman G Jr, Oliver R, Odejinmi F. A twelve year analysis of non-tubal ectopic pregnancies: do the clinical manifestations and risk factor for these rare pregnancies differ from those of tubal pregnancies? Gynecol Surg. 2016; 13:103–109.
28. Casikar I, Condous G. How to effectively diagnose ectopic pregnancy using ultrasound? Expert Rev Obstet Gynecol. 2013; 8:493–495.
29. Female internal genital organs. Moore KL, Dalley AF, Agur AM, editors. Clinically oriented anatomy. 7th ed. Philadelphia (PA): Wolters Kluwer/Lippincott Williams & Wilkins Health;2014. p. 382–399.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download