Abstract
Objective
Methods
Results
Conclusion
Figures and Tables
Table 1
Clinical characteristics of the in vitro fertilization cycles in women with diminished ovarian reserve
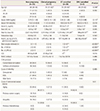
Data are presented as median (range), mean±standard deviation, or number (%).
ET, embryo transfer; OPU, oocyte pickup; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; E2, estradiol; AFC, antral follicle count; Gn, gonadotropin; hCG, human chorionic gonadotropin; MII, metaphase II; PN, pronuclei; CES, cumulative embryo score; TMSC, total motile sperm count; COH, controlled ovarian hyperstimulation; EMS, endometriosis.
a)Comparison with the non-pregnancy group by post hoc analysis; b)Kruskal-Wallis test was performed, and data are expressed as median (range); c)Comparison with the clinical pregnancy group by post hoc analysis.
Table 2
Factors that affect clinical pregnancy in women with diminished ovarian reserve

Model 1 was adjusted for the total AFC, number of 2PN, and cause of diminished ovarian reserve. Model 2 was adjusted for the same variables used in model 1, except for AFC, which was substituted with serum AMH level.
OR, odds ratio; CI, confidence interval; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; AFC, antral follicle count; E2, estradiol; MII, metaphase II; PN, pronuclei; CES, cumulative embryo score; EMS, endometriosis.
Table 3
Factors that influence cycle cancellation in women with diminished ovarian reserve
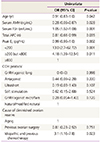
Table 4
Adjusted ORs of the risk factors of cycle cancellation

Model 1 of cycle cancellation was adjusted for peak estradiol (<200 pg/dL, ≥200 but <800 pg/dL, and ≥800 pg/dL), serum follicle-stimulating hormone level, total antral follicle count, controlled ovarian hyperstimulation protocol (gonadotropin releasing hormone agonist long, antagonist, ultrashort, soft stimulation, gonadotropin releasing hormone agonist microflare, and natural/modified natural); Model 2 of cycle cancellation was adjusted for the same variables used in model 1, except for serum follicle-stimulating hormone level, which was substituted with serum anti-Müllerian hormone level.
OR, odds ratio; CI, confidence interval.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download