This article has been corrected. See "Erratum: Author correction" in Volume 60 on page 621.
Abstract
Objective
This study aimed to evaluate the efficacy and safety of pegylated liposomal doxorubicin (PLD) with or without carboplatin in Korean patients with recurrent ovarian cancer (ROC), fallopian tube, or primary peritoneal cancer.
Methods
This retrospective study included 52 patients with ROC, fallopian tube, or primary peritoneal cancer who received PLD (50 mg/m2) between 1st December 2014 and 31th July 2016.
Results
The mean number of chemotherapy cycles was 3.8 (range, 2 to 9) in the PLD monotherapy group and 7 (range, 2 to 13) in the PLD combined with carboplatin (PLD-C) group. In overall response rates and clinical beneficial rates, PLD monotherapy group shows 5.0% and 17.5%, and PLD-C group shows 33.3% and 75.0%. The mean progression-free survival (PFS) was 5 and 13 months in the PLD monotherapy and PLD-C groups, respectively. At 6 months after treatment initiation, absence of disease progression was confirmed in 6 (15%) and 10 (83.3%) patients in the PLD monotherapy and PLD-C groups. Hematological adverse events (e.g., neutropenia and thrombocytopenia) were more common in the PLD-C group (P<0.001, P=0.004). The incidence of anemia and non-hematological adverse events, including mucositis, hand-foot syndrome, and allergic reactions, was similar in both groups.
Conclusion
This study demonstrated the efficacy and safety of PLD monotherapy and PLD-C combination in Korean patients with ROC. This study would be helpful to consider the degree of worry about side effects and treatment expectations after treatment. Further retrospective studies with larger samples are required to confirm the efficacy of PLD monotherapy in Asian patients with platinum-resistant ROC.
Ovarian cancer is the most challenging gynecological malignancy to be treated and the fifth most common cause of cancer-related deaths among females [1]. Only 40–60% of patients with ovarian cancer can achieve complete remission after staging surgery followed by paclitaxel with carboplatin chemotherapy [2]. There are several chemotherapy regimens for recurrent ovarian cancer (ROC), which should be selected according to platinum sensitivity [3]. Treatment of platinum-sensitive ROC is based on combination therapies, including platinum agents (carboplatin [Paraplatin®; Bristol-Myers Squibb, Princeton, NJ, USA] and cisplatin [Platinol®; Bristol-Myers Squibb]). However, combination chemotherapy has additional concerns of increased adverse effects such as bone marrow depression or nephrotoxicity. In platinum-resistant ROC, single-agent chemotherapy is considered.
Doxorubicin has been used for ROC; however, its use is limited owing to adverse effects, particularly cardiotoxicity, which increases with cumulative doses [4]. Hence, pegylated liposomal doxorubicin (PLD; Caelyx®; TTY Biopharm Co., Ltd., Taipei, Taiwan), with an increased circulating half-life and a better toxicity profile, including cardiotoxicity and myelosuppression, through its liposomal envelope leads to low doses of free doxorubicin in the normal tissue [5]. The trial of Caelyx in Platinum Sensitive Ovarian (CALYPSO) patients conducted by the Gynecological Cancer InterGroup (GCIG) showed that PLD combined with carboplatin (PLD-C) had better progression-free survival (PFS) and similar overall survival (OS) compared with paclitaxel (Taxol®; Bristol-Myers Squibb) and carboplatin in platinum-sensitive ROC [6]. In addition, PLD-C had less severe toxicities, particularly alopecia, compared with paclitaxel and carboplatin [7]. However, the incidence of mucositis and hand-foot syndrome (HFS, palmar-plantar erythrodysesthesia) is high with PLD-C. Certain studies on platinum-resistant ROC have suggested that PLD monotherapy has similar efficacy compared with topotecan or gemcitabine [8910]. PLD monotherapy showed more frequent HFS and less frequent neutropenia compared with gemcitabine (Gemzar®; Eli Lilly and Company, Indianapolis, IN, USA).
Although PLD has been approved and commercially available in Korea since December 2014, there are no related articles of PLD experience in Korean population yet. Although there was a study in Japan on patients with ROC, the study was phase II clinical trial and most of the patients received only 1 or 2 previous chemotherapy regimens [11]. Patients with advanced ROC with more than 3 previous chemotherapy regimens are more likely to use PLD monotherapy after developing platinum resistance; hence, there is a need for data regarding the effectiveness and safety of PLD monotherapy in real-world clinical settings. To date, there are no retrospective studies on PLD monotherapy in Asian patients. Thus, we aimed to evaluate the efficacy and safety of PLD treatment in Korean patients with ROC.
The study protocol was approved by the Institutional Review Board at the Asan Medical Center (IRB No. 2015-0722). A retrospective chart review of consecutively treated patients who begin received either PLD or PLD-C therapy for the treatment of ROC at Asan Medical Center, Seoul, Korea from 1st December 2014 to 20th March 2015 was conducted. Patients were divided on the basis of the platinum-free interval (PFI) from the last chemotherapy (<6 vs. ≥6 months). For patients with PFI of ≥6 months, PLD-C was administered as treatment for platinum-sensitive ROC. A dose of 50 mg/m2 PLD was administered as an intravenous infusion in PLD and PLD-C group. In case of PLD-C, patients received carboplatin add at a dose of 5 areas under curve (AUC). The treatment was repeated every 28 days and was delayed in case of unacceptable toxicities. Only those patients who received treatment at least 2 times were included in the analysis. Chemotherapy was basically administered for 6 consecutive cycles and then continued until disease progression. Cancer antigen 125 (CA-125) levels were monitored at each cycle. Tumor status was assessed using computed tomography after every 3 cycles of chemotherapy and could be assessed before time if any signs of disease progression were observed. Objective response rate (ORR; complete response [CR] + partial response [PR]) was assessed according to the Response Evaluation Criteria in Solid Tumor (RECIST criteria version 1.1) [12]. CR was the disappearance of all target lesions and PR was >30% decrease in the sum of the longest diameter of the target lesion; the mean of both these values was considered as ORR. Stable disease (SD) included lesions that were insufficient to constitute as PR or progressive disease (PD). Overall response rate was assessed using ORR and CA-125 response according to the GCIG criteria [13]. Clinical benefit rate (CBR) was defined as CR, PR, or SD. It was impossible to assess OS because the follow-up period was too short.
Patients were monitored for toxicities and allergic reactions following treatment initiation. All patients received additional monitoring for HFS, mucositis, and bone marrow depression at 1 week after every administration in the outpatient clinic. Adverse events were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE version 4.0). When grade 3 or 4 neutropenia was occurred, granulocyte stimulating factor was given to the patient. In patients with ≥ grade 2 HFS or mucositis, chemotherapy was delayed for up to 2 weeks or until the lesions resolved to grade 0 or 1. In addition, patients with a history of ≥ grade 3 toxicities received 25% dose reduction. The baseline characteristics and adverse events of both groups were compared using χ2 tests. The Kaplan-Meier method was used for PFS analysis. All statistical analyses were performed using the SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA).
A total of 52 patients were eligible for the study criteria and the total follow-up period was 1,108 months (range, 18 to 23 months). All patients were evaluated for response rates. Patient characteristics are summarized in Table 1. The mean age was 57.4 years (range, 32 to 74 years); 40 patients had received 155 cycles of PLD monotherapy, and 12 patients had received 84 cycles of PLD-C therapy. Patient's age, initial stage, histological subtype, largest lesion, and pretreatment CA-125 levels were similar in both groups. The treatment-free interval were 3.7 months in PLD monotherapy group and 10.7 months in the PLD-C group, respectively (P=0.002). And more patients in the PLD monotherapy group had received more than three chemotherapy regimens in the past compared with the PLD-C group (P=0.001).
The subgroup responses according to the chemotherapy regimen administered are summarized in Table 2. All patients were eligible for response evaluation. The mean number of chemotherapy cycles administered was 3.8 (range, 2 to 9) in the PLD monotherapy group and 7 (range, 2 to 13) in the PLD-C group. ORR and CBR were 5.0% and 17.5% in the PLD monotherapy group and 33.3% and 75.0% in the PLD-C group, respectively. Kaplan-Meier curves for PFS in each group are presented in Fig. 1. The mean PFS was 5 months in the PLD monotherapy group and 13 months in the PLD-C group. At 6 months after treatment initiation, no disease progression was noted in 6 (15%) patients of the PLD monotherapy group and 10 (83.3%) patients of the PLD-C group. Moreover, 27 (67.5%) patients of the PLD monotherapy group and one (8.3%) patient of the PLD-C group died during the treatment period.
Adverse events according to chemotherapy regimens are summarized in Table 3. Hematological adverse events such as neutropenia and thrombocytopenia were more common in the PLD-C group (P<0.001 and P=0.004). The incidence of anemia and non-hematological adverse events such as mucositis, HFS, and allergic reactions was similar in both groups. The prevalence of mucositis and HFS was 10.3% and 9.0% in the PLD monotherapy group and 16.7% and 14.3% in the PLD-C group, respectively. Three (7.5%) patients from the PLD monotherapy group and 6 (50%) from the PLD-C group received dose reduction in chemotherapy because of adverse events; furthermore, 1 patient discontinued PLD monotherapy because of severe continuous HFS above grade 3.
PLD-C therapy exhibited better therapeutic effects than PLD monotherapy, which of course caused by the differences of baseline predictive factors on survival, such as treatment-free interval, number of previous chemotherapy regimens, and platinum sensitivity. Approximately half of the patients of the PLD monotherapy group had previously received more than 3 chemotherapy regimens, and treatment was initiated at <1 month after failure of the previous chemotherapy.
In the PLD monotherapy group, ORR and CBR were 5.0% and 17.5%, respectively. Table 4 presents a comparison of efficacy of PLD monotherapy with different studies [111415], and the results of this study are poorer than those of other studies. While the average number of previous chemotherapy regimens of other studies was less than 2, the patients in the present study had an average of 3.5 previous chemotherapy regimens; the marked difference in this basic factor appears to have affected the response rate. However, in actual clinical situations, PLD monotherapy is administered to patients with advanced disease; thus, several patients have a history of more than 3 previous chemotherapy regimens. Despite the differences in response rates, there was no significant difference in the time to progression among the aforementioned studies from 4 to 5 months. Considering these results, the effectiveness of PLD appears to be largely dependent on the pretreatment differences in the baseline predictive factors and does not appear to be considerably influenced by racial differences.
PLD therapy is clinically administered at various doses in the range of 30–50 mg/m2; thus, the available evidence should be carefully interpreted because the efficacy and adverse effects may vary. The present study was conducted with a maximum tolerated dose of 50 mg/m2, but certain studies have reported similar efficacy and lower toxicity with doses of 40 mg/m2 [1617]. According to the results of this study, the efficacy of PLD monotherapy in platinum-resistant ROC does not appear to be more than expected; therefore, it might be worthwhile to consider a lower dose of treatment that can reduce toxicity without significantly decreasing the efficacy, and a comparative study of the therapeutic effect and side effects of each dose in Asian women is required.
In combination therapy with carboplatin, dose reductions were more frequent and the incidence of hematological adverse events such as neutropenia and thrombocytopenia was higher. Anemia appears to have been largely unaffected by the use of prophylactic iron supplements during treatment. There was no significant difference was observed in the incidence of non-hematological adverse events such as mucositis or HFS; this may be due to the use of the same dose of PLD in both groups. HFS is considered to be the dose-limiting toxicity of PLD therapy [18]. Only one patient with PLD monotherapy discontinued treatment after the fifth injection because of severe continuous HFS above grade 3; she rested afterward, and disease progression was observed 3 months after discontinuation. Other patients who had mucositis or HFS showed improvement in symptoms and continued treatment after delayed dosing and dose reduction.
This study had some limitations of note. It was a retrospective study and there could be a bias by its nature. Because the PLD treatment was only recently approved in Korea, the number of PLD-C groups was small because of PLD is often used in patients who had already acquired platinum resistance. In this study, left ventricular ejection fraction was not routinely checked for all patients. Cardiotoxicity is a major concern with anthracyclines; however, the liposomal envelope of PLD leads to low doses of free doxorubicin in the normal tissue and lowers its toxicity profile [1920]. Several studies have reported low cardiotoxicity with PLD; thus, monitoring of cardiotoxicity is not necessarily required for patients receiving PLD therapy who do not have any cardiac symptoms [212223]. Moreover, none of the study patients complained any signs or symptoms of cardiac abnormality.
In conclusion, this study demonstrated the efficacy and safety of PLD monotherapy and PLD-C in Korean patients with ROC. To the best of our knowledge, this is the first retrospective study on PLD monotherapy in Asian patients. PLD monotherapy is widely used as a salvage therapy with anti-tumor activity in patients with platinum-resistant ROC. According to the results of this study, the efficacy of PLD monotherapy in platinum-resistant ROC appears to be largely dependent on pretreatment differences in baseline predictive factors. This study would be helpful to consider the degree of worry about side effects and treatment expectations after the treatment. Further additional retrospective studies with larger patient samples are needed to confirm the efficacy of PLD monotherapy in Asian patients with platinum-resistant ROC.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.

2. Morgan RJ Jr, Alvarez RD, Armstrong DK, Burger RA, Chen LM, Copeland L, et al. Ovarian cancer, version 2.2013. J Natl Compr Canc Netw. 2013; 11:1199–1209. PMID: 24142821.

3. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24(Suppl 6):vi24–vi32. PMID: 24078660.

4. Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010; 115:155–162. PMID: 20016174.

5. Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014; 10:853–858. PMID: 25579518.

6. Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010; 28:3323–3329. PMID: 20498395.

7. Mahner S, Meier W, du Bois A, Brown C, Lorusso D, Dell'Anna T, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Eur J Cancer. 2015; 51:352–358. PMID: 25534295.

8. Gordon AN, Tonda M, Sun S, Rackoff W. Doxil Study 30-49 Investigators. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004; 95:1–8. PMID: 15385103.

9. Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007; 25:2811–2818. PMID: 17602086.

10. Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008; 26:890–896. PMID: 18281662.

11. Katsumata N, Fujiwara Y, Kamura T, Nakanishi T, Hatae M, Aoki D, et al. Phase II clinical trial of pegylated liposomal doxorubicin (JNS002) in Japanese patients with mullerian carcinoma (epithelial ovarian carcinoma, primary carcinoma of fallopian tube, peritoneal carcinoma) having a therapeutic history of platinum-based chemotherapy: a Phase II Study of the Japanese Gynecologic Oncology Group. Jpn J Clin Oncol. 2008; 38:777–785. PMID: 18927230.

12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.

13. Vergote I, Rustin GJ, Eisenhauer EA, Kristensen GB, Pujade-Lauraine E, Parmar MK, et al. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J Natl Cancer Inst. 2000; 92:1534–1535. PMID: 10995813.
14. Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000; 18:3093–3100. PMID: 10963637.

15. Mayer C, Brucker J, Schuetz F, Domschke C, Bechstein S, Heil J, et al. Efficacy and toxicity profile of pegylated liposomal doxorubicin in patients with advanced ovarian cancer. Arch Gynecol Obstet. 2016; 294:123–129. PMID: 26498757.

16. Nakayama M, Kobayashi H, Takahara T, Nishimura Y, Fukushima K, Yoshizawa K. A comparison of overall survival with 40 and 50mg/m(2) pegylated liposomal doxorubicin treatment in patients with recurrent epithelial ovarian cancer: propensity score-matched analysis of real-world data. Gynecol Oncol. 2016; 143:246–251. PMID: 27612976.
17. Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. Phase 2 trial of liposomal doxorubicin (40 mg/m(2)) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000; 78:369–372. PMID: 10985896.

18. Lotem M, Hubert A, Lyass O, Goldenhersh MA, Ingber A, Peretz T, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000; 136:1475–1480. PMID: 11115157.

19. Working PK, Dayan AD. Pharmacological-toxicological expert report. CAELYX. (Stealth liposomal doxorubicin HCl). Hum Exp Toxicol. 1996; 15:751–785. PMID: 8880211.
20. Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997; 54(Suppl 4):15–21.
21. Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B, et al. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi's sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol. 1998; 9:711–716. PMID: 9739435.

22. O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004; 15:440–449. PMID: 14998846.
23. Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000; 11:1029–1033. PMID: 11038041.
Fig. 1
PFS in each group. PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; PLD-C, pegylated liposomal doxorubicin combined with carboplatin.
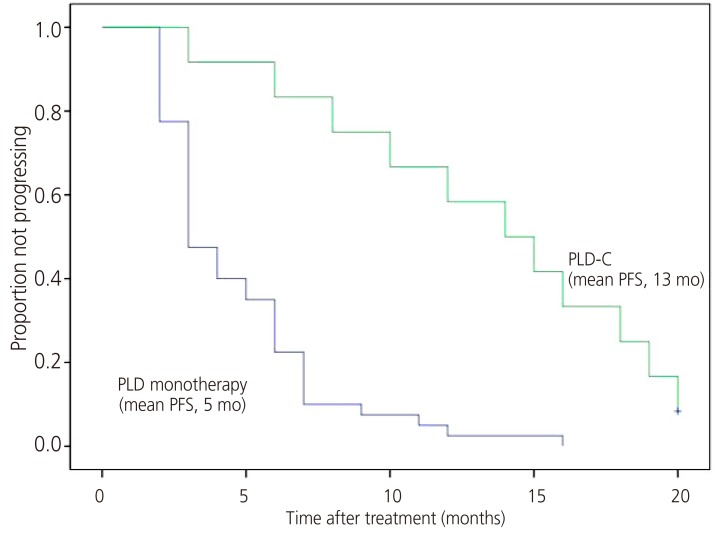
Table 1
Baseline characteristics of subgroups according to the chemotherapy regimens
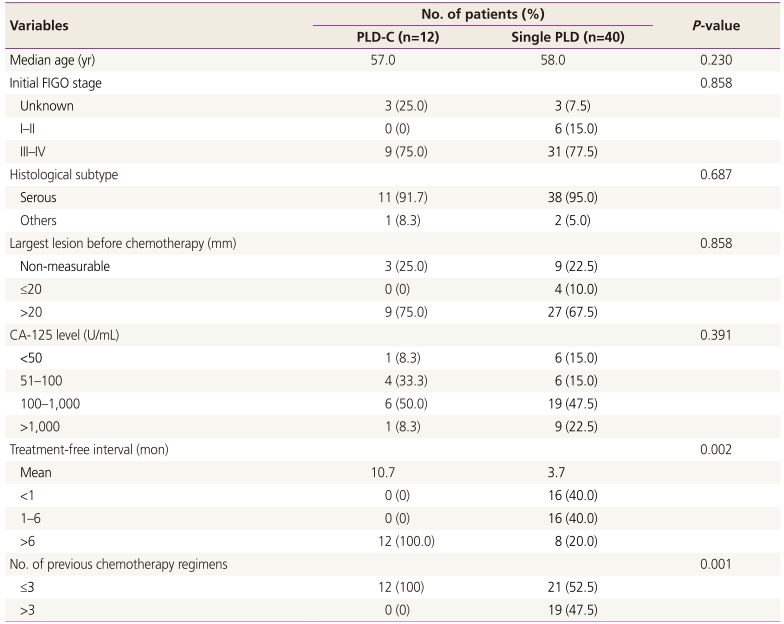
Table 2
Response of subgroups according to the chemotherapy regimens
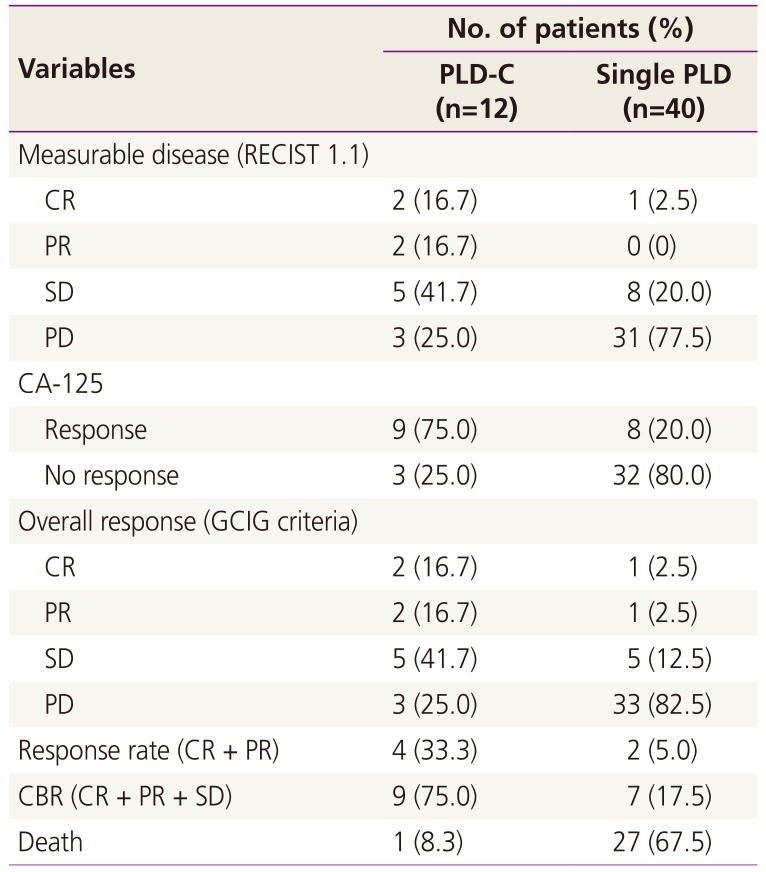
PLD, pegylated liposomal doxorubicin; PLD-C, pegylated liposomal doxorubicin combined with carboplatin; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; CA-125, cancer antigen 125; GCIG, Gynecologic Cancer Intergroup; CBR, clinical benefit rate.
Table 3
Adverse events according to the chemotherapy regimens
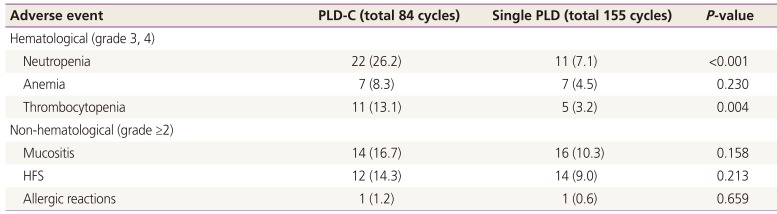
Table 4
Comparison of efficacy of PLD monotherapy with that reported in other studies
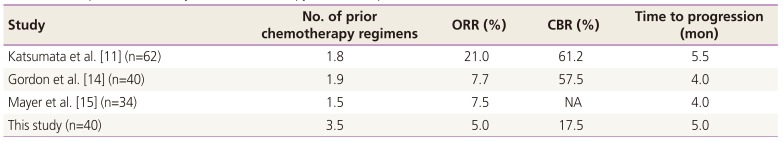
| Study | No. of prior chemotherapy regimens | ORR (%) | CBR (%) | Time to progression (mon) |
|---|---|---|---|---|
| Katsumata et al. [11] (n=62) | 1.8 | 21.0 | 61.2 | 5.5 |
| Gordon et al. [14] (n=40) | 1.9 | 7.7 | 57.5 | 4.0 |
| Mayer et al. [15] (n=34) | 1.5 | 7.5 | NA | 4.0 |
| This study (n=40) | 3.5 | 5.0 | 17.5 | 5.0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download