Introduction
Type, routes, dose, and interval of administration
Table 1
Type, route, dose, and interval of progesterone supplement therapy for prevention of preterm birth
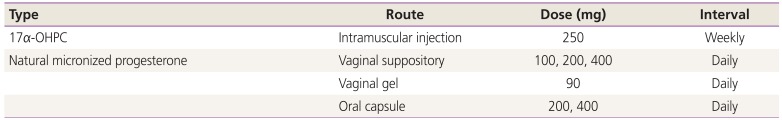
| Type | Route | Dose (mg) | Interval |
|---|---|---|---|
| 17α-OHPC | Intramuscular injection | 250 | Weekly |
| Natural micronized progesterone | Vaginal suppository | 100, 200, 400 | Daily |
| Vaginal gel | 90 | Daily | |
| Oral capsule | 200, 400 | Daily |
Summary of previous studies based on the indications
1. History of PTB
Table 2
Summary of randomized controlled trials of progesterone supplement therapy for prevention of PTB in women with history of PTB
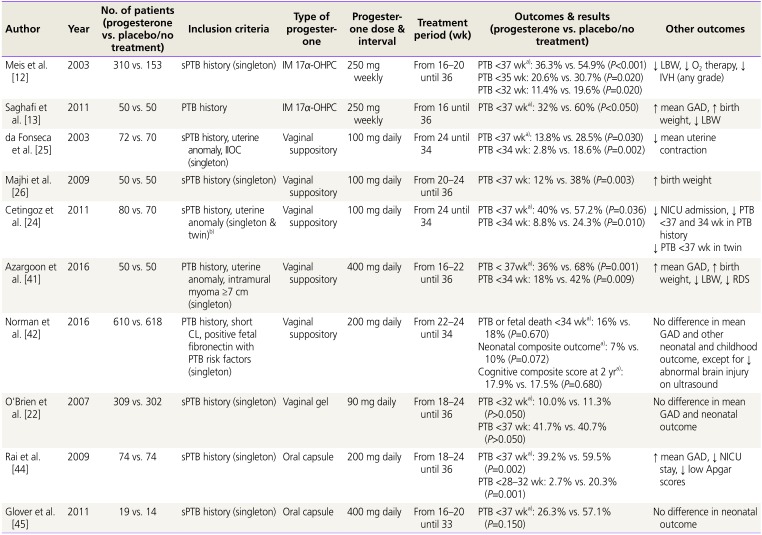
| Author | Year | No. of patients (progesterone vs. placebo/no treatment) | Inclusion criteria | Type of progesterone | Progesterone dose & interval | Treatment period (wk) | Outcomes & results (progesterone vs. placebo/no treatment) | Other outcomes |
|---|---|---|---|---|---|---|---|---|
| Meis et al. [12] | 2003 | 310 vs. 153 | sPTB history (singleton) | IM 17α-OHPC | 250 mg weekly | From 16–20 until 36 | PTB <37 wka): 36.3% vs. 54.9% (P<0.001) | ↓ LBW, ↓ O2 therapy, ↓ IVH (any grade) |
| PTB <35 wk: 20.6% vs. 30.7% (P=0.020) | ||||||||
| PTB <32 wk: 11.4% vs. 19.6% (P=0.020) | ||||||||
| Saghafi et al. [13] | 2011 | 50 vs. 50 | PTB history | IM 17α-OHPC | 250 mg weekly | From 16 until 36 | PTB <37 wka): 32% vs. 60% (P<0.050) | ↑ mean GAD, ↑ birth weight, ↓ LBW |
| da Fonseca et al. [25] | 2003 | 72 vs. 70 | sPTB history, uterine anomaly, IIOC (singleton) | Vaginal suppository | 100 mg daily | From 24 until 34 | PTB <37 wka): 13.8% vs. 28.5% (P=0.030) | ↓ mean uterine contraction |
| PTB <34 wk: 2.8% vs. 18.6% (P=0.002) | ||||||||
| Majhi et al. [26] | 2009 | 50 vs. 50 | sPTB history (singleton) | Vaginal suppository | 100 mg daily | From 20–24 until 36 | PTB <37 wk: 12% vs. 38% (P=0.003 ) | ↑ birth weight |
| Cetingoz et al. [24] | 2011 | 80 vs. 70 | sPTB history, uterine anomaly (singleton & twin)b) | Vaginal suppository | 100 mg daily | From 24 until 34 | PTB <37 wka): 40% vs. 57.2% (P=0.036) |
↓ NICU admission, ↓ PTB <37 and 34 wk in PTB history ↓ PTB <37 wk in twin |
| PTB <34 wk: 8.8% vs. 24.3% (P=0.010) | ||||||||
| Azargoon et al. [41] | 2016 | 50 vs. 50 | PTB history, uterine anomaly, intramural myoma ≥7 cm (singleton) | Vaginal suppository | 400 mg daily | From 16–22 until 36 | PTB < 37wka): 36% vs. 68% (P=0.001) | ↑ mean GAD, ↑ birth weight, ↓ LBW, ↓ RDS |
| PTB <34 wk: 18% vs. 42% (P=0.009) | ||||||||
| Norman et al. [42] | 2016 | 610 vs. 618 | PTB history, short CL, positive fetal fibronectin with PTB risk factors (singleton | Vaginal suppository | 200 mg daily | From 22–24 until 34 | PTB or fetal death <34 wka): 16% vs. 18% (P=0.670) | No difference in mean GAD and other neonatal and childhood outcome, except for ↓ abnormal brain injury on ultrasound |
| Neonatal composite outcomea): 7% vs. 10% (P=0.072) | ||||||||
| Cognitive composite score at 2 yra): 17.9% vs. 17.5% (P=0.680) | ||||||||
| O'Brien et al. [22] | 2007 | 309 vs. 302 | sPTB history (singleton) | Vaginal gel | 90 mg daily | From 18–24 until 36 | PTB <32 wka): 10.0% vs. 11.3% (P>0.050) | No difference in mean GAD and neonatal outcome |
| PTB <37 wk: 41.7% vs. 40.7% (P>0.050) | ||||||||
| Rai et al. [44] | 2009 | 74 vs. 74 | sPTB history (singleton) | Oral capsule | 200 mg daily | From 18–24 until 36 | PTB <37 wka): 39.2% vs. 59.5% (P=0.002) | ↑ mean GAD, ↓ NICU stay, ↓ low Apgar scores |
| PTB <28–32 wk: 2.7% vs. 20.3% (P=0.001) | ||||||||
| Glover et al. [45] | 2011 | 19 vs. 14 | sPTB history (singleton) | Oral capsule | 400 mg daily | From 16–20 until 33 | PTB <37 wka): 26.3% vs. 57.1% (P=0.150) | No difference in neonatal outcome |
PTB, preterm birth; sPTB, spontaneous preterm birth; IM, intramuscular; 17α-OHPC, 17-alpha hydroxyprogesterone caproate; LBW, low birth weight; IVH, intraventricular hemorrhage; GAD, gestational age at delivery; IIOC, incompetent internal os of cervix; NICU, neonatal intensive care unit; RDS, respiratory distress syndrome; CL, cervical length.
a)Primary outcome; b)A total of 67 twin pregnancies (39 in the progesterone group and 28 in the placebo group) were included.
1) 17α-OHPC
2) Vaginal natural micronized progesterone suppository
3) Vaginal natural micronized progesterone gel
4) Oral natural micronized progesterone capsule
2. Short CL
Table 3
Summary of randomized controlled trials of progesterone supplement therapy for prevention of PTB in in women with short CL
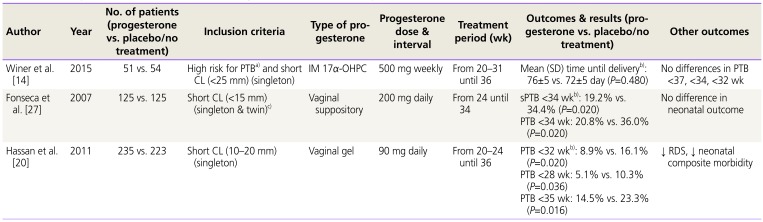
| Author | Year | No. of patients (progesterone vs. placebo/no treatment) | Inclusion criteria | Type of progesterone | Progesterone dose & interval | Treatment period (wk) | Outcomes & results (progesterone vs. placebo/no treatment) | Other outcomes |
|---|---|---|---|---|---|---|---|---|
| Winer et al. [14] | 2015 | 51 vs. 54 | High risk for PTBa) and short CL (<25 mm) (singleton) | IM 17α-OHPC | 500 mg weekly | From 20–31 until 36 | Mean (SD) time until deliveryb): 76±5 vs. 72±5 day (P=0.480) | No differences in PTB <37, <34, <32 wk |
| Fonseca et al. [27] | 2007 | 125 vs. 125 | Short CL (<15 mm) (singleton & twin)c) | Vaginal suppository | 200 mg daily | From 24 until 34 | sPTB <34 wkb): 19.2% vs. 34.4% (P=0.020) | No difference in neonatal outcome |
| PTB <34 wk: 20.8% vs. 36.0% (P=0.020) | ||||||||
| Hassan et al. [20] | 2011 | 235 vs. 223 | Short CL (10–20 mm) (singleton) | Vaginal gel | 90 mg daily | From 20–24 until 36 | PTB <32 wkb): 8.9% vs. 16.1% (P=0.020) | ↓ RDS, ↓ neonatal composite morbidity |
| PTB <28 wk: 5.1% vs. 10.3% (P=0.036) | ||||||||
| PTB <35 wk: 14.5% vs. 23.3% (P=0.016) |
PTB, preterm birth; CL, cervical length; IM, intramuscular; 17α-OHPC, 17-alpha hydroxyprogesterone caproate; SD, standard deviation; sPTB, spontaneous preterm birth; RDS, respiratory distress syndrome.
a)History of PTB or cervical surgery or uterine malformation or prenatal diethylstilbestrol exposure; b)Primary outcome; c)A total of 24 twin pregnancies (11 in the progesterone group and 13 in the placebo group) were included.
1) 17α-OHPC
2) Vaginal natural micronized progesterone suppository
3) Vaginal natural micronized progesterone gel
3. Twin pregnancy
Table 4
Summary of randomized controlled trials of progesterone supplement therapy for prevention of PTB in women with twin pregnancy
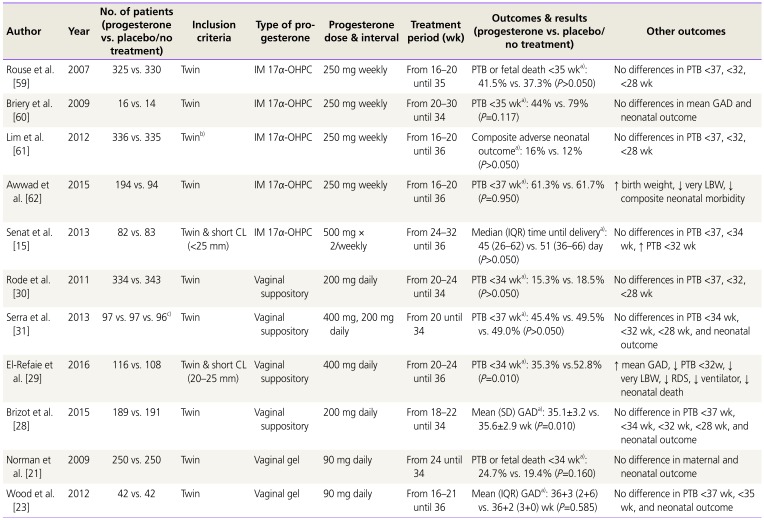
| Author | Year | No. of patients (progesterone vs. placebo/no treatment) | Inclusion criteria | Type of progesterone | Progesterone dose & interval | Treatment period (wk) | Outcomes & results (progesterone vs. placebo/no treatment) | Other outcomes |
|---|---|---|---|---|---|---|---|---|
| Rouse et al. [59] | 2007 | 325 vs. 330 | Twin | IM 17α-OHPC | 250 mg weekly | From 16–20 until 35 | PTB or fetal death <35 wka): 41.5% vs. 37.3% (P>0.050) | No differences in PTB <37, <32, <28 wk |
| Briery et al. [60] | 2009 | 16 vs. 14 | Twin | IM 17α-OHPC | 250 mg weekly | From 20–30 until 34 | PTB <35 wka): 44% vs. 79% (P=0.117) | No differences in mean GAD and neonatal outcome |
| Lim et al. [61] | 2012 | 336 vs. 335 | Twinb) | IM 17α-OHPC | 250 mg weekly | From 16–20 until 36 | Composite adverse neonatal outcomea): 16% vs. 12% (P>0.050) | No differences in PTB <37, <32, <28 wk |
| Awwad et al. [62] | 2015 | 194 vs. 94 | Twin | IM 17α-OHPC | 250 mg weekly | From 16–20 until 36 | PTB <37 wka): 61.3% vs. 61.7% (P=0.950) | ↑ birth weight, ↓ very LBW, ↓ composite neonatal morbidity |
| Senat et al. [15] | 2013 | 82 vs. 83 | Twin & short CL (<25 mm) | IM 17α-OHPC | 500 mg ×2/weekly | From 24–32 until 36 | Median (IQR) time until deliverya): 45 (26–62) vs. 51 (36–66) day (P>0.050) | No differences in PTB <37, <34 wk, ↑ PTB <32 wk |
| Rode et al. [30] | 2011 | 334 vs. 343 | Twin | Vaginal suppository | 200 mg daily | From 20–24 until 34 | PTB <34 wka): 15.3% vs. 18.5% (P>0.050) | No differences in PTB <37, <32, <28 wk |
| Serra et al. [31] | 2013 | 97 vs. 97 vs. 96c) | Twin | Vaginal suppository | 400 mg, 200 mg daily | From 20 until 34 | PTB <37 wka): 45.4% vs. 49.5% vs. 49.0% (P>0.050) | No differences in PTB <34 wk, <32 wk, <28 wk, and neonatal outcome |
| El-Refaie et al. [29] | 2016 | 116 vs. 108 | Twin & short CL (20–25 mm) | Vaginal suppository | 400 mg daily | From 20–24 until 36 | PTB <34 wka): 35.3% vs.52.8% (P=0.010) | ↑ mean GAD, ↓ PTB <32w, ↓ very LBW, ↓ RDS, ↓ ventilator, ↓ neonatal death |
| Brizot et al. [28] | 2015 | 189 vs. 191 | Twin | Vaginal suppository | 200 mg daily | From 18–22 until 34 | Mean (SD) GADa): 35.1±3.2 vs. 35.6±2.9 wk (P=0.010) | No difference in PTB <37 wk, <34 wk, <32 wk, <28 wk, and neonatal outcome |
| Norman et al. [21] | 2009 | 250 vs. 250 | Twin | Vaginal gel | 90 mg daily | From 24 until 34 | PTB or fetal death <34 wka): 24.7% vs. 19.4% (P=0.160) | No difference in maternal and neonatal outcome |
| Wood et al. [23] | 2012 | 42 vs. 42 | Twin | Vaginal gel | 90 mg daily | From 16–21 until 36 | Mean (IQR) GADa): 36+3 (2+6) vs. 36+2 (3+0) wk (P=0.585) | No difference in PTB <37 wk, <35 wk, and neonatal outcome |
PTB, preterm birth; IM, intramuscular; 17α-OHPC, 17-alpha hydroxyprogesterone caproate; GAD, gestational age at delivery; LBW, low birth weight; CL, cervical length; IQR, interquartile range; SD, standard deviation; RDS, respiratory distress syndrome.
a)Primary outcome; b)Women with history of sPTB were excluded; c)Progesterone 400 vs. progesterone 200 vs. placebo.
1) 17α-OHPC
2) Vaginal natural micronized progesterone suppository
3) Vaginal natural micronized progesterone gel
4. Preterm labor and premature rupture of membranes
Table 5
Summary of randomized controlled trials of progesterone supplement therapy for prevention of PTB in women with preterm labor or preterm premature rupture of membranes
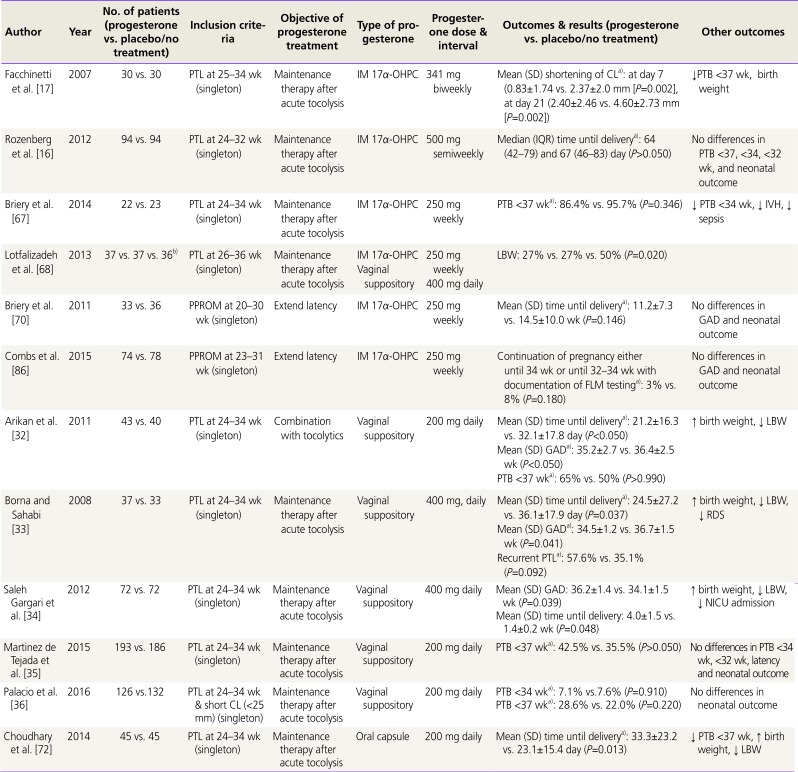
| Author | Year | No. of patients (progesterone vs. placebo/no treatment) | Inclusion criteria | Objective of progesterone treatment | Type of progesterone | Progesterone dose & interval | Outcomes & results (progesterone vs. placebo/no treatment) | Other outcomes |
|---|---|---|---|---|---|---|---|---|
| Facchinetti et al. [17] | 2007 | 30 vs. 30 | PTL at 25–34 wk (singleton) | Maintenance therapy after acute tocolysis | IM 17α-OHPC | 341 mg biweekly | Mean (SD) shortening of CLa): at day 7 (0.83±1.74 vs. 2.37±2.0 mm [P=0.002], at day 21 (2.40±2.46 vs. 4.60±2.73 mm [P=0.002]) | ↓PTB <37 wk, birth weight |
| Rozenberg et al. [16] | 2012 | 94 vs. 94 | PTL at 24–32 wk (singleton) | Maintenance therapy after acute tocolysis | IM 17α-OHPC | 500 mg semiweekly | Median (IQR) time until deliverya): 64 (42–79) and 67 (46–83) day (P>0.050) | No differences in PTB <37, <34, <32 wk, and neonatal outcome |
| Briery et al. [67] | 2014 | 22 vs. 23 | PTL at 24–34 wk (singleton) | Maintenance therapy after acute tocolysis | IM 17α-OHPC | 250 mg weekly | PTB <37 wka): 86.4% vs. 95.7% (P=0.346) | ↓ PTB <34 wk, ↓ IVH, ↓ sepsis |
| Lotfalizadeh et al. [68] | 2013 | 37 vs. 37 vs. 36b) | PTL at 26–36 wk (singleton) | Maintenance therapy after acute tocolysis |
IM 17α-OHPC Vaginal suppository |
250 mg weekly 400 mg daily |
LBW: 27% vs. 27% vs. 50% (P=0.020) | |
| Briery et al. [70] | 2011 | 33 vs. 36 | PPROM at 20–30 wk (singleton) | Extend latency | IM 17α-OHPC | 250 mg weekly | Mean (SD) time until deliverya): 11.2±7.3 vs. 14.5±10.0 wk (P=0.146) | No differences in GAD and neonatal outcome |
| Combs et al. [86] | 2015 | 74 vs. 78 | PPROM at 23–31 wk (singleton) | Extend latency | IM 17α-OHPC | 250 mg weekly | Continuation of pregnancy either until 34 wk or until 32–34 wk with documentation of FLM testinga): 3% vs. 8% (P=0.180) | No differences in GAD and neonatal outcome |
| Arikan et al. [32] | 2011 | 43 vs. 40 | PTL at 24–34 wk (singleton) | Combination with tocolytics | Vaginal suppository | 200 mg daily | Mean (SD) time until deliverya): 21.2±16.3 vs. 32.1±17.8 day (P<0.050) | ↑ birth weight, ↓ LBW |
| Mean (SD) GADa): 35.2±2.7 vs. 36.4±2.5 wk (P<0.050) | ||||||||
| PTB <37 wka): 65% vs. 50% (P>0.990) | ||||||||
| Borna and Sahabi [33] | 2008 | 37 vs. 33 | PTL at 24–34 wk (singleton) | Maintenance therapy after acute tocolysis | Vaginal suppository | 400 mg, daily | Mean (SD) time until deliverya): 24.5±27.2 vs. 36.1±17.9 day (P=0.037) | ↑ birth weight, ↓ LBW, ↓ RDS |
| Mean (SD) GADa): 34.5±1.2 vs. 36.7±1.5 wk (P=0.041) | ||||||||
| Recurrent PTLa): 57.6% vs. 35.1% (P=0.092) | ||||||||
| Saleh Gargari et al. [34] | 2012 | 72 vs. 72 | PTL at 24–34 wk (singleton) | Maintenance therapy after acute tocolysis | Vaginal suppository | 400 mg daily | Mean (SD) GAD: 36.2±1.4 vs. 34.1±1.5 wk (P=0.039) | ↑ birth weight, ↓ LBW, ↓ NICU admission |
| Mean (SD) time until delivery: 4.0±1.5 vs. 1.4±0.2 wk (P=0.048) | ||||||||
| Martinez de Tejada et al. [35] | 2015 | 193 vs. 186 | PTL at 24–34 wk (singleton) | Maintenance therapy after acute tocolysis | Vaginal suppository | 200 mg daily | PTB <37 wka): 42.5% vs. 35.5% (P>0.050) | No differences in PTB <34 wk, <32 wk, latency and neonatal outcome |
| Palacio et al. [36] | 2016 | 126 vs.132 | PTL at 24–34 wk & short CL (<25 mm) (singleton) | Maintenance therapy after acute tocolysis | Vaginal suppository | 200 mg daily | PTB <34 wka): 7.1% vs.7.6% (P=0.910) | No differences in neonatal outcome |
| PTB <37 wka): 28.6% vs. 22.0% (P=0.220) | ||||||||
| Choudhary et al. [72] | 2014 | 45 vs. 45 | PTL at 24–34 wk (singleton) | Maintenance therapy after acute tocolysis | Oral capsule | 200 mg daily | Mean (SD) time until deliverya): 33.3±23.2 vs. 23.1±15.4 day (P=0.013) | ↓ PTB <37 wk, ↑ birth weight, ↓ LBW |
PTB, preterm birth; PTL, preterm labor; IM, intramuscular; 17α-OHPC, 17-alpha hydroxyprogesterone caproate; SD, standard deviation; CL, cervical length; IQR, interquartile range; IVH, intraventricular hemorrhage; LBW, low birth weight; PPROM, preterm premature rupture of membranes; GAD, gestational age at delivery; FLM, fetal lung maturity; RDS, respiratory distress syndrome; NICU, neonatal intensive care unit.
a)Primary outcome; b)IM progesterone vs. vaginal progesterone vs. placebo.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download