Abstract
Disorders of sex development (DSD) are congenital conditions characterized by atypical development of chromosomal, gonadal, and phenotypic sex. 46, XY DSD can result from disorders of testicular development or disorders of androgen synthesis/action. Prophylactic gonadectomy should be considered in patients with 46, XY DSD because of the increased risk of gonadal malignancy. We report two rare cases of 46, XY DSD, including XY pure gonadal dysgenesis and complete androgen insensitivity syndrome, who underwent a prophylactic gonadectomy.
Disorders of sex development (DSD) are congenital conditions characterized by atypical development of chromosomal, gonadal, and phenotypic sex. DSD is divided into three groups according to the chromosomal component: 46, XX DSD; 46, XY DSD; and sex chromosomal DSD. 46, XY DSD is rare but may occur at any point in the sexual differentiation pathway, which is a complex process of gene interactions, androgen synthesis, and hormone regulation by the interaction of ligands with their corresponding receptors [1]. The diagnosis of patients with 46, XY DSD is mainly clinical and usually identified during investigation for primary amenorrhea or delayed puberty. Prophylactic gonadectomy should be considered for patients with DSD who have Y chromosomes, due to an increased risk of gonadal malignancy. We report two rare cases of 46, XY DSD patients who underwent a prophylactic gonadectomy due to the risk of developing a gonadal malignancy: one case is XY pure gonadal dysgenesis associated with impairment of testicular development and the other is complete androgen insensitivity syndrome (CAIS) related to androgen receptor inability to respond to androgens but otherwise normal testicular development and androgen synthesis. We also discuss the pathophysiological processes and the optimal timing of gonadectomy with the evidence on risk of neoplasia in patients with 46, XY DSD.
A 17-year-old girl was referred to our clinic for evaluation of primary amenorrhea and delayed puberty. She had no relevant past medical or family history. Her weight was 56 kg, height was 168 cm, and body mass index was 19.8 kg/m2. The patient had small breasts that were developed inadequately (Tanner breast stage 2) and no pubic hair (Tanner pubic hair stage 1). Examination of the genitalia revealed that the patient had normal female external genitalia with an intact hymen and a vagina with a depth of 5 cm (Fig. 1A). Transabdominal ultrasonography and magnetic resonance imaging (MRI) revealed a small uterus, measuring about 15 mm in length; however, neither ovaries nor testes were visible. Laboratory evaluation showed markedly elevated levels of follicle-stimulating hormone (65.08 mIU/mL) and very low estradiol levels (less than 5 pg/mL). The serum level of luteinizing hormone was 21.31 mIU/mL and that of testosterone was 3 ng/dL, which was within the normal range for women of that age. A karyotype was obtained and showed a normal male karyotype 46, XY. We planned to perform prophylactic laparoscopic gonadectomy on the basis of the diagnosis of XY gonadal dysgenesis. Laparoscopic exploration revealed the presence of bilateral streak gonads with a small uterus and normal appearing fallopian tubes (Fig. 1B-D). The patient underwent laparoscopic bilateral gonadectomy and salpingectomy (Fig. 1E, F). Pathological examination of the specimens showed bilateral streak ovaries but no evidence of Sertoli cell-like components or malignant components (Fig. 1G). The patient was prescribed cyclic estrogen and progesterone replacement therapy.
A 23-year-old woman underwent evaluation for primary amenorrhea. She had a surgical history of inguinal hernia at the age of 8 years. Her weight was 62 kg, height was 160 cm, and body mass index was 24.2 kg/m2. The patient had no axillary hair, Tanner stage 3 breast development, and Tanner stage 1 pubic hair growth. Inspection of her external genitalia showed normal appearing labia majora and minora. However, a short, blind vaginal pouch was detected with a depth of 4 cm. Inguinal masses were palpable on both sides and identified in MRI as testis-like gonads located at the inguinal canal. Transvaginal ultrasonography and MRI showed the absence of a uterus, fallopian tubes, and ovaries. Laboratory evaluation revealed follicle-stimulating hormone, luteinizing hormone and estradiol serum levels of 21.2 mIL/mL, 22.6 mIL/mL, and 46.5 pg/mL, respectively. Serum testosterone was elevated at 1,032 ng/dL, and chromosomal analysis confirmed a normal male 46, XY karyotype. The patient was diagnosed with CAIS. Gonadectomy was recommended because of the malignant transformation potential of occult testicular elements. We performed bilateral gonadectomy through the inguinal approach, which allowed easy access to the testes in both inguinal canals. The surgery was uncomplicated, and pathological examination of the gonads showed Leydig cell hyperplasia and only Sertoli cells in the seminiferous tubules at both sides (Fig. 1H, I). The patient was started on estrogen therapy for breast development and bone health.
46, XY pure gonadal dysgenesis, previously known as Swyer syndrome, can be caused by mutation in the sex-determining region on Y (SRY) gene located on the distal short arm of the Y chromosome (Yp11.3) [1]. Mutations in other genes such as SOX9, DAX1, WT-1 and SF1 that are involved in the regulation of SRY expression or act as a transcription activator downstream of SRY in the testis-determining pathway may also result in XY pure gonadal dysgenesis [2]. The mutations involved in testicular development result in the development of undifferentiated streak gonads, which do not produce antimüllerian hormone or androgens [1]. Consequently, the vagina, cervix, uterus, and fallopian tubes develop, and external genitalia are those of females because of the absence of androgen action (Fig. 2).
Unlike the dysgenetic gonads in XY pure gonadal dysgenesis, individuals with CAIS have normal testes, and Leydig cells in the testicle normally produce testosterone. However, androgen action is deficient because of mutations in the androgen receptor gene [1]. Consequently, the Wolffian duct spontaneously regresses in the absence of androgens and cannot differentiate into the epididymis and vas deferens. And the Sertoli cells in the testes release antimüllerian hormone in the normal manner and induce the regression of müllerian structures [1]. Therefore, patients with CAIS have a short, blind pouch vagina without a uterus, ovaries, or fallopian tubes (Fig. 2).
In patients with CAIS, the testes may be located anywhere along the path of embryonic testicular descent; however, they are more commonly located in the inguinal canals or labia majora [3]. Deeb and Hughes [4] reported that more than half of patients with CAIS presented with inguinal hernia; half of these were bilateral and one-third contained gonads.
Careful history taking and physical examination are of the utmost importance in the diagnostic process of patients with 46, XY DSD. In both our cases, patients presented with female external genitalia. However, the patient with CAIS was distinguished by adequate breast development, probably due to the action of estrogens unopposed by the action of androgens. Also, MRI can be an effective diagnostic tool for patients with 46, XY DSD, particularly in identifying a hypoplastic uterus in XY pure gonadal dysgenesis and locating cryptorchid testes in CAIS cases. In addition, high serum levels of testosterone also help to distinguish CAIS from XY pure gonadal dysgenesis.
The clinical management of an XY female patient generally includes prophylactic gonadectomy to prevent malignant transformation, appropriate hormone replacement therapy (HRT), and psychological counseling.
Individuals whose karyotypes contain a Y cell are predisposed to gonadal ridge tumors, including gonadoblastomas, dysgerminomas, yolk sac tumors, and choriocarcinomas [5]. Prophylactic gonadectomy is recommended for these patients to eliminate the risk of potential malignant changes of the gonads [5]. When considering the timing of gonadectomy in patients with XY pure gonadal dysgenesis, early gonadectomy is advised because of the high risk of malignant potential, which is estimated to be 46% to 75% [1]. In our case of XY pure gonadal dysgenesis, the patient underwent laparoscopic bilateral gonadectomy and salpingectomy soon after diagnosis. Patients with XY pure gonadal dysgenesis can become pregnant through in vitro fertilization using donor oocytes [6]. Compared with XY pure gonadal dysgenesis, gonadectomy is advised after pubertal development is completed in CAIS cases because of the low risk of malignancy before puberty and the fact that unopposed testicular estrogen induces breast development [7]. In our case of CAIS, the patient underwent prophylactic gonadectomy after the pubertal development, and there was no evidence of malignancy in gonads.
After gonadectomy has been performed, exogenous HRT should be provided for XY female patients to initiate, mature, and maintain secondary sexual characteristics [8]. Prevention of coronary heart disease and osteoporosis is an additional benefit of estrogen therapy [8]. For individuals with XY pure gonadal dysgenesis, cyclic estrogen and progestogen therapy initiated after the completion of estrogen-induced breast development will prevent endometrial hyperplasia that may result from unopposed estrogen stimulation [8]. In both our cases, patients received HRT, which will be continued until they reach 50 years of age.
Psychological counseling for the patient and their parents is an important element of management for patients with 46, XY DSD. In general, most individuals with 46, XY DSD have a completely female phenotype; therefore, female gender identity may be reinforced. When patients experience difficulty with penetration due to vaginal hypoplasia, a functional vagina may be considered for normal sexual satisfaction by progressive vaginal dilation, traditional vaginoplasty or laparoscopic Vecchietti method [9].
To our knowledge, there have been few report describing the clinical imaging findings in patients with 46, XY DSD. Imaging findings including female external genitalia, streak gonads, and uterus in our case would be help for clinicians to assess patients with 46, XY DSD.
Figures and Tables
Fig. 1
Clinical findings of a patient with XY pure gonadal dysgenesis. (A) Normal external female genitalia with an intact hymen. (B) Small uterus (asterisk). (C) Left streak ovary (arrow) and fallopian tube (arrowhead). (D) Right streak ovary (arrow) and fallopian tube (arrowhead). (E) Left streak ovary and fallopian tube transected with LigaSure. (F) Right streak ovary and fallopian tube transected with LigaSure. (G) Microscopic finding of a streak ovary in a patient with XY pure gonadal dysgenesis, showing simple cuboidal lining of modified mesothelial cells in the superficial epithelium of the streak ovary. The underlining stroma is composed of spindled cells arranged in parallel or a storiform pattern, which are characteristic findings of ovaria stroma (H&E, ×100). (H) Gross finding of inguinal testes in a patient with complete androgen insensitivity syndrome. (I) Microscopic finding of inguinal testes in a patient with complete androgen insensitivity syndrome, showing immature testicular tubules with a thick hyalinized basement membrane (H&E, ×100).
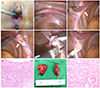
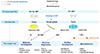
Fig. 2
A schematic diagram of different pathways involved in sexual development of patients presenting with XY pure gonadal dysgenesis and complete androgen insensitivity syndrome (CAIS). SF1, steroidogenic factor 1; WT1, wilms' tumor 1; SRY, sex-determining region Y; WNT4, wingless-type MMTV integration site family member 4; FOXL2, forkhead transcription factor L2; DAX1, dosage sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1; RSPO1, R-spondin 1; SOX9, SRY-related HMG box 9; AMH, antimüllerian hormone; T, testosterone; DHT, dihydrotestosterone; E2, estradiol; AR, androgen receptor.
References
1. Michala L, Creighton SM. The XY female. Best Pract Res Clin Obstet Gynaecol. 2010; 24:139–148.
2. Jorgensen PB, Kjartansdottir KR, Fedder J. Care of women with XY karyotype: a clinical practice guideline. Fertil Steril. 2010; 94:105–113.
3. Oakes MB, Eyvazzadeh AD, Quint E, Smith YR. Complete androgen insensitivity syndrome: a review. J Pediatr Adolesc Gynecol. 2008; 21:305–310.
4. Deeb A, Hughes IA. Inguinal hernia in female infants: a cue to check the sex chromosomes? BJU Int. 2005; 96:401–403.
5. Cools M, Stoop H, Kersemaekers AM, Drop SL, Wolffenbuttel KP, Bourguignon JP, et al. Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J Clin Endocrinol Metab. 2006; 91:2404–2413.
6. Plante BJ, Fritz MA. A case report of successful pregnancy in a patient with pure 46,XY gonadal dysgenesis. Fertil Steril. 2008; 90:2015.
7. Deans R, Creighton SM, Liao LM, Conway GS. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin Endocrinol (Oxf). 2012; 76:894–898.
8. Baker VL, Schillings WJ. Amenorrhea. In : Berek JS, Novak E, editors. Berek & Novak's gynecology. 15th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2012. p. 1035–1062.
9. Ismail-Pratt IS, Bikoo M, Liao LM, Conway GS, Creighton SM. Normalization of the vagina by dilator treatment alone in complete androgen insensitivity syndrome and Mayer-Rokitansky-Kuster-Hauser syndrome. Hum Reprod. 2007; 22:2020–2024.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download