Abstract
Primary vulva malignancy is a rare gynecologic malignancy. Most of them are squamous cell carcinomas and adenocarcinomas are much less common. Intestinal type is a rare variant of primary adenocarcinoma of the vulva. It histologically resembles mucinous colonic carcinomas. Origin from cloacal remnants has been suggested but remains speculative. A 64-year-old woman was referred to our clinic with a 1-month history of an itching vulva mass. An incisional biopsy was performed at other hospital and disclosed adenocarcinoma of intestinal type. Extensive workups were performed to detect other underlying carcinomas but revealed nothing abnormal. She underwent wide local excision without lymph node dissection for a primary vulva carcinoma. She received no adjuvant therapy and has been free from recurrent disease for 12 months after surgery. The authors report a rare case and review the relevant literature.
Primary vulva cancer is a rare gynecologic malignancy and represents 3% to 5% of malignancies of the female genital track [1]. About 90% of primary vulva malignancies are squamous cell carcinomas and adenocarcinomas are much less common. Most of these lesions originated from Bartholin's gland or is associated with Paget's disease [2]. Intestinal type adenocarcinoma is a rare variant of primary adenocarcinoma of the vulva and only a few cases have been reported. It histologically resembles mucinous colonic carcinomas. Origin from cloacal remnants has been suggested but remains speculative. The authors report a rare case and review the relevant literature.
A 64-year-old woman, gravida 3, para 3, was referred to our clinic with a 1-month history of an itching vulva mass. Clinical examination demonstrated 5 cm×3 cm sized nodular mass was located on the right posterior part of the major labium but the uterus was small and the adnexa not palpable. Cervicovaginal Pap smear was negative and transvaginal ultrasonography revealed a small myoma. An incisional biopsy was performed at other hospital and diagnosed as adenocarcinoma of intestinal type. At that time, immunohistochemistry reveled positive reaction for CEA, cytokeratin (CK) 20, CK 7, CDX 2, p53 and p16. Tumor markers such as CA 125, CEA, and squamous cell carcinoma antigen were all within normal limit. Extensive workups were performed to detect other underlying carcinoma. Gastroduodenoscopy, colonoscopy, and cystoscopy revealed nothing abnormal. Positron emission tomography–computed tomography (PET-CT) did not demonstrate any other local or systemic carcinomas.
She underwent wide local excision without lymph node dissection for a primary vulva carcinoma. On gross examination the vulva tissue widely excised was 4 cm×3 cm×1.8 cm and the skin surface was protruded (Fig. 1A). The cut surface showed a tumor mass, 3.5 cm×2.7 cm, and appeared to be dermal invasion with infiltration of abundant mucus material. On microscopic finding the tumor was a well differentiated invasive adenocarcinoma having focal papillary pattern with the vulvar ulcerating the surface epithelium. The neoplastic glands that reveled intestinal type mucinous epithelium with hyperchromatic nuclei, goblet cells and intracytoplasmic mucin (Fig. 1B, C). There was large amount of mucin pool present. The tumor invaded deep dermis with 10 mm in depth and 14 mm in width. Mitotic figures were frequently seen. Lymphatic or vascular invasion was absent. The surgical margin was free from the tumor. Immunohistochemistry was performed repeatedly in excision tumor, and showed diffuse positive in CEA, CK 20, CK 7, p53 and p16INK4a (Fig. 1D-F). This intestinal type carcinoma was confined to vulva, without invasion to vagina or anus. We, in conjunction with the clinical information, diagnosed this case as mucinous adenocarcinoma, intestinal type, arising from the vulva. She received no adjuvant therapy and has been free from recurrent disease for 12 months after surgery.
Primary vulva carcinoma affects postmenopausal women and the vast majority of tumors are squamous cell carcinomas. Primary adenocarcinoma of the vulva is rare and can arise from normally situated glands in vulva or from ectopic tissues or embryonic remnants. Lulenski and Naji [3] first described the mucin-secreting adenocarcinoma of the vulva and it was originated from the Bartholin's gland. Other possible sites are sweat glands, Skene's gland, minor vestibular glands, aberrant mammary tissue or endometriotic implants [4]. Intestinal type is a rare variant of primary mucinous adenocarcinoma of the vulva, which histologically resembles mucinous colonic carcinomas. Only small number of cases has been reported and a search of the literature was conducted. The clinical features and managements of reported cases including our cases are summarized in Table 1 [24567891011].
Metastatic tumors account for 8% of vulva tumors and the common sites are the cervix, the endometrium, kidney and urethra. Most patients with vulvar metastases have advanced primary tumors and the primary lesion and the vulva metastasis are diagnosed simultaneously in about one forth [12]. So meticulous research is mandatory to detect other local or systemic cancers. Due to its histological similarity, metastatic workups including gastroscopy, colonoscopy, CT or PET-CT have been performed.
Immunochemical analysis can be used to differentiate the origin of the tumor; estrogen receptor, progesterone receptor, and gross cystic disease fluid protein for mammary gland and endometrial carcinoma, CA 125 for ovary cancer, CK 20, villin and CA 19-9 for adenocarcinoma of gastrointestinal tract, and transcription intermediary factor 1, and napsin A for lung adenocarcinoma. In our case, the intestinal type mucinous carcinoma appears to be arising from vulva epithelium. Immunohistochemistry showed diffuse positive reaction for CEA and CK 20, that suggests intestinal type mucinous carcinoma, but CK 7, which is usually diffuse positive reaction in Müllerian ducts of the female genital tract, revealed diffuse positive reaction. From this result we deduced that our case is a primary intestinal type mucinous carcinoma of the vulva epithelium. Immunohistochemical staining for p53 and p16INK4a showed diffuse positive and focal positive reactions, respectively. If the case is related with human papillomavirus infection, p16INK4a will show diffuse positive reaction. However, our case may not be related with human papillomavirus infection because of focal positive in p16INK4a. Additionally, other medical diagnostic workups such as tumor markers (CA 125, CEA, and squamous cell carcinoma), endoscopy (gastroduodenoscopy, colonoscopy, and cystoscopy) and PET-CT showed no abnormality. Therefore, we confirmed the intestinal type mucinous carcinoma is the primary vulva tumor.
Since this tumor shows the same morphology and immunohistochemistry as many adenocarcinomas of the large bowl and surrounding epithelium is similar to the large bowel mucosa, this tumor has been suggested to originate from the cloacal remnants. It could be misplaced [5] or aberrant tissue [6] or might be a normal feature of that area [7].
During embryogenesis, the labium majora develop from the labioscrotal folds and the labia minora develop from the urethral folds. Both structures are closely associated with cloacal development and can undergo malignant transformation similar to a primary adenocarcinoma of the colon [56]. But the origin of this tumor is uncertain.
The clinical behavior of this unusual malignant tumors appeared indolent. Among 12 patients, only one patient recurred and died of disease. Multiple metastatic lesions in liver, pelvic and aortic nodes, and rectum were detected 36 months after initial surgery. She received chemotherapy and achieved complete remission. But she developed diffuse recurrent disease after six months and died of disease. The authors did not know whether that was a real recurrence from primary vulva carcinoma or a secondary metachronous intestinal carcinoma [4].
Traditionally, radical vulvectomy and bilateral dissection of the groin and pelvic lymph nodes was the standard treatment for the patients with early vulva cancer. But it was associated with significant surgical morbidity and psychosexual sequelae so the modern approach for patients with operable vulva cancer has been individualized [13]. Radical local excision is a safe surgical option, if with sufficient tumor-free surgical margin. Among 12 patients, six patients did not undergo radical vulvectomy. So the same surgical approaches can be advocated in the mucinous adenocarcinoma of the vulva.
In summary, intestinal type is a rare variant of primary adenocarcinoma of the vulva and only a few cases have been reported. So there are no standardized protocols for diagnosis, clinical course, and treatment. It histologically resembles mucinous colonic carcinomas and meticulous workups are needed before confirmation. We show the patient has no evidence of disease at 12 months after initial surgery without adjuvant therapy, thus providing useful information to better inform and establish the future treatment modality for intestinal type mucinous adenocarcinoma of the vulva.
Figures and Tables
Fig. 1
(A) The vulva reveals a reddish dome-like cutaneous mass. (B) The tumor shows an infiltrative mucinous carcinoma and predominant mucin pools. (C) The mucinous carcinoma is intestinal types with presence of characteristically colonic goblet cells (inset) and intracytoplasmic mucin. (D) On immunohistochemical staining the tumor cells show diffuse positive reaction for CEA, (E) cytokeratin 20, and (F) cytokeratin 7.
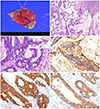
Table 1
Overview of intestinal-type mucinous adenocarcinoma of the vulva in the literature

| Author (year) | Age (yr) | Operation | Adjuvant therapy | Follow-up (mo) | Outcome |
|---|---|---|---|---|---|
| Tiltman et al. (1978) [5] | 50 | MRV & LND | None | 12 | NED |
| Kennedy et al. (1993) [6] | 54 | RV & LND | None | 120 | NED |
| 63 | WLE | None | 48 | NED | |
| Ghamande et al. (1995) [2] | 67 | RV & LND | None | Unknown | Unknown |
| Willen et al. (1999) [7] | 57 | WLE | None | 26 | NED |
| Zaidi et al. (2001) [8] | 43 | MRV & LND | None | 18 | NED |
| Rodriguez et al. (2001) [9] | 69 | WLE | None | 36 | NED |
| Dube et al. (2004) [10] | 64 | WLE | None | 4.5 | NED |
| Cormio et al. (2012) [4] | 59 | RV & LND | None | 54 | DOD |
| 42 | RV & LND | None | 39 | NED | |
| Sui et al. (2016) [11] | 43 | WLE | Chemotherapy | 24 | NED |
| Current study | 64 | WLE | None | 12 | NED |
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56:106–130.
2. Ghamande SA, Kasznica J, Griffiths CT, Finkler NJ, Hamid AM. Mucinous adenocarcinomas of the vulva. Gynecol Oncol. 1995; 57:117–120.
3. Lulenski CR, Naji AF. Mucin-secreting adenocarcinoma of Bartholin gland: report of a case. Obstet Gynecol. 1964; 24:542–544.
4. Cormio G, Carriero C, Loizzi V, Gissi F, Leone L, Putignano G, et al. “Intestinal-type” mucinous adenocarcinoma of the vulva: a report of two cases. Eur J Gynaecol Oncol. 2012; 33:433–435.
5. Tiltman AJ, Knutzen VK. Primary adenocarcinoma of the vulva originating in misplaced cloacal tissue. Obstet Gynecol. 1978; 51:30s–33s.
6. Kennedy JC, Majmudar B. Primary adenocarcinoma of the vulva, possibly cloacogenic: a report of two cases. J Reprod Med. 1993; 38:113–116.
7. Willen R, Bekassy , Carlen B, Bozoky B, Cajander S. Cloacogenic adenocarcinoma of the vulva. Gynecol Oncol. 1999; 74:298–301.
8. Zaidi SN, Conner MG. Primary vulvar adenocarcinoma of cloacogenic origin. South Med J. 2001; 94:744–746.
9. Rodriguez A, Isaac MA, Hidalgo E, Marquez B, Nogales FF. Villoglandular adenocarcinoma of the vulva. Gynecol Oncol. 2001; 83:409–411.
10. Dube V, Veilleux C, Plante M, Tetu B. Primary villoglandular adenocarcinoma of cloacogenic origin of the vulva. Hum Pathol. 2004; 35:377–379.
11. Sui Y, Zou J, Batchu N, Lv S, Sun C, DU J, et al. Primary mucinous adenocarcinoma of the vulva: a case report and review of the literature. Mol Clin Oncol. 2016; 4:545–548.
12. Dehner LP. Metastatic and secondary tumors of the vulva. Obstet Gynecol. 1973; 42:47–57.
13. Hacker NF, Berek JS, Lagasse LD, Nieberg RK, Leuchter RS. Individualization of treatment for stage I squamous cell vulvar carcinoma. Obstet Gynecol. 1984; 63:155–162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download