Abstract
Objective
We evaluated the learning curve for external cephalic version (ECV) using learning curve-cumulative sum (LC-CUSUM) analysis.
Methods
This was a retrospective study involving 290 consecutive cases between October 2013 and March 2017. We evaluated the learning curve for ECV on nulli and over para 1 group using LC-CUSUM analysis on the assumption that 50% and 70% of ECV procedures succeeded by description a trend-line of quadratic function with reliable R2 values.
Results
The overall success rate for ECV was 64.8% (188/290), while the success rate for nullipara and over para 1 groups was 56.2% (100/178) and 78.6% (88/112), respectively. ‘H’ value, that the actual failure rate does not differ from the acceptable failure rate, was −3.27 and −1.635 when considering ECV success rates of 50% and 70%, respectively. Consequently, in order to obtain a consistent 50% success rate, we would require 57 nullipara cases, and in order to obtain a consistent 70% success rate, we would require 130 nullipara cases. In contrast, 8 to 10 over para 1 cases would be required for an expected success rate of 50% and 70% on over para 1 group.
Conclusion
Even a relatively inexperienced physician can experience success with multipara and after accumulating experience, they will manage nullipara cases. Further research is required for LC-CUSUM involving several practitioners instead of a single practitioner. This will lead to the gradual implementation of standard learning curve guidelines for ECV.
Breech presentation occurs in 3% to 4% of single pregnancies, reaching term. In 2012, the cesarean delivery rate in the United States of America was 32.8%, compared to 36.0% in Korea [1]. External cephalic version (ECV) is an obstetric procedure, which aims to turn a fetus into a cephalic presentation by moving the maternal abdomen. A Cochrane review reported that ECV at full-term gestation (>37 weeks) reduces the likelihood of a non-cephalic presentation at birth and thus, the need for a cesarean section [2]. On this basis, ECV should be recommended for all women with a breech fetus at term when there are no contraindications [3].
In some countries, including Korea, however, ECV has been a relatively unfamiliar procedure until recently and there are few physicians with the skills required to carry out this technique [1]. This skill depends on a range of factors, including individual skills, environmental circumstances and institutional factors and the time taken to achieve the required level of skill can thus influence the clinical outcomes of patients [4]. Although there have been numerous studies investigating factors which might influence success rate of ECV, such as amniotic fluid index, parity, and type of breech but very few of these have specifically investigated the learning curve ECV; only one paper published involving 80 cases, has investigated ECV [5].
The ‘learning curve’ is defined as an improvement in performance over time or with increasing experience or training. We considered the possibility of the existence of a learning curve when we first started using a simple ‘moving average curve’ when our experience reached approximately 200 cases. But because of limitation its objectivity and quantification, we proposed ‘cumulative sum (CUSUM) analysis’ that is a sequential analysis tool that was initially used in industrial settings for quality control purposes. It can allow one to judge when an individual's performance has achieved a predefined level of competence [4]. More recently, various fields, including medical fields, have applied quality control procedures to assess the learning curves of trainees and monitor the introduction of innovative technologies [6]. The slope of CUSUM expresses the tendency of the learning achievement while the aspect of the slope showing stabilization represents a breakthrough in learning. This system has the advantage of allowing for easier visual verification of when the evaluation of a specific technique reaches a certain level [7]. Furthermore, this allows us to convey to patients our consistent proficiency in ECV success during counseling sessions in the clinic. Therefore, in this paper, we aimed to evaluate the learning curve for ECV using CUSUM and to determine consistent proficiency for the ECV for nullipara and over para 1.
This retrospective observational study was gone through by the institutional review board of Cheil General Hospital (CGH-IRB-2017-14). The study involved 290 consecutive cases of ECV, performed between October 2013 and March 2017 by a single practitioner. Demographic factors were collected for each subject from medical records, including age, parity, maternal body weight, fetal estimated weight, AFI, type of breech, location of placenta, fixation status of the presenting part, whether any other procedure was involved (e.g., amniotic fluid infusion), contraction status in admission, presence of myoma, and buttock elevation status. Our contraindications for ECV were multiple pregnancy, oligohydramnios, antepartum hemorrhage, rupture of membranes, any contraindication to vaginal delivery, utero-placental insufficiency, intrauterine growth restriction, preeclampsia, and non-reassuring fetal monitoring pattern.
All patients agreed to undergo the procedure, and they were informed of the probability of failure and the risks associated with the procedure, including fetal distress and emergency cesarean delivery. Patients were admitted after fasting for eight hours and laboratory tests were conducted in preparation for cesarean sections, including complete blood cell count, blood typing, electrolytes, liver function test, and urinalysis. We also arranged the preventive mutual cooperation between an anesthesiologist and the operation team. The ECV procedure was discontinued immediately if the fetal heart rate showed a non-reassuring pattern, or if the patient complained of an intolerable discomfort, despite the use of epidural anesthesia.
All ECVs were performed in the delivery unit of Cheil General Hospital & Women's Healthcare Center with the patient lying horizontally on a bed. Just before the ECV, we assessed the contraction pattern and fetal heart beat variability by continuous fetal heart rate monitoring for more than 30 minutes. We then administered more than 30 minutes of tocolysis using an intravenous infusion of ritodrine hydrochloride (Lavopa) and epidural anesthesia. Fetal presentation was confirmed by performing transabdominal ultrasonography of the fetal spinous side. The ECV procedure team consisted of a maximum of four members, including a main supervisor (clinical operator), sonographer (resident), and two helpers, including one nurse. As starting this procedure, the sonographer (resident) checked the location of fetal head, spine and fetal heart beat. Main clinical operator stood the other side from ultrasonography to gaze the patient’s foot side. And in the process, if the fetus is descending or engaging too much in pelvic cavity, one team member stood the same side besides to operator to gaze the patient's face and push to upward from symphysis pubis for free float the fetus. And one other member, if necessary, stood the opposite side from operator and push for prevent to oust of fetus. Sonographer moved the probe along with the fetal head moving and checked the fetal heart beat when finishing one time trial of ECV.
We tried to avoid exceeding 10 minutes during a single trial and the maximum number of continuous trials was less than five. If the initial ECV failed, we performed another ECV trial after the recommendation of amnioinfusion only after with the patient's agreement. After a successful procedure, the patient was placed in a lateral decubitus position and continuous fetal monitoring was continued for an additional 3 hours; ritodrine and anesthesia infusion were stopped. After confirmation of a reassuring fetal heartbeat and the lack of regular contractions, the patient was discharged to their home. The following day, non-stress test and an official general checkup was performed. Following ECV, we defined the procedure as a success if the presentation showed a fetal head without positioning of any extremities, such as the hands or feet, in the same plane as the fetal head.
We assumed that an ECV failure rate of more than 70% by a single practitioner would be unacceptable, and that this value should be fixed [8]. Accordingly, we analyzed our data and plotted appropriate graphs for two groups: 1) the learning curve of a 50% success rate (close to average); and 2) the learning curve of a 70% consistent success rate (considered as expert). Each of these groups was then categorized into three subgroups based on their patients, as follows: overall, nullipara, and over para 1 (=para 1 and beyond). Since the overall cumulative success rate may be influenced by execution order, we used the overall CUSUM score to only determine general tendency.
There were 178 cases of nullipara, 94 cases of para 1, 17 cases of para 2, and 1 case of para 3; overall there were 112 cases over para 1 so, total 290 cases were included in our study. Among that, total 188 cases were succeeded and 102 cases were failed. The mean maternal age was 33.4 years that included 34.1 years in success (n=188) group and 32.3 years in fail (n=102) group. The age, gravida, parity were statistically significant to succeed. And the mean gestational age was 37.4 weeks, AFI was 10.8 cm and maternal body mass index was 25.3 kg/m2 (Table 1). And 287 patients received ECV under epidural anesthesia, while three patients received ECV without epidural anesthesia. Ritodrine was used for tocolysis for 247 patients but not in the remaining 43 patients.
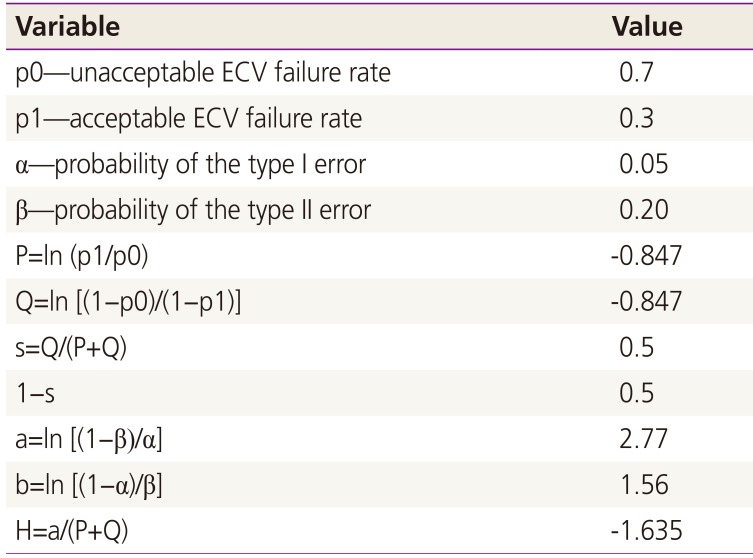
For CUSUM analysis, four parameters were defined (Tables 2, 3): the unacceptable failure rate (p0), the acceptable failure rate (p1), the type I error rate (α), and the type II error rate (β) [4]. The probabilities of α and β were set at 0.05 and 0.20, respectively. The results of CUSUM analysis are presented in a chart with case numbers plotted on the X-axis and the corresponding CUSUM score on the Y-axis (Figs. 1, 2, 3), which allows performance over consecutive procedures to be readily visualized.
At a defined point (t), the basic statistics for calculating CUSUM analysis were as follows:
In this expression, C0 is 0, and Yt represents a successful or non-successful procedure (failure=1, success=0) at the point referred to as t. Y0 is the calculated value for the assumed failure rate considered by researchers. Thus, if a consecutive procedure is successful, then the CUSUM score at point t will decrease by as much as Y0 from the previous value Ct-1. If the procedure fails, then the CUSUM score will add 1−Y0 onto the previous score.
Hence, success is represented by a downward slope on the graph, while failure is represented by an upward slope. If the line crosses the lower decision limit (H), this indicates that the actual failure rate does not differ from the acceptable failure rate. The equations shown in Tables 2 and 3 were used to calculate the CUSUM score. The null hypothesis was ‘unskilled and inexperienced status’ while the alternative hypothesis was ‘skillful and experienced status.’ Therefore, if the CUSUM penetrates the limit value, then this would be statistically significant and mean that the alternative hypothesis should be accepted and that we should dismiss the null hypothesis [7].
When the acceptable and unacceptable ECV failure rate is 50%, and 70%, respectively, we can simplify the equation as follows:
When the acceptable and unacceptable ECV failure rate is 30% and 70%, respectively, we can simplify the equation as follows:
We entered data from the polynomial curve of CUSUM, and the value of R2 using a trend line, into Excel (Microsoft, Redmond, WA, USA) and carried out statistical analysis using the Student's t-test on SPSS ver 17.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
The overall ECV success rate was 64.8% (188/290 cases), while the success rate was 56.2% (100/178 cases) for nullipara patients and 78.6% (88/112 cases) for over para 1 patients. However, considering the entire 290 cases, the success rate of the most recent 100 cases, regardless of parity, was 80%, and it reached a 100% success rate over the most recent 20 cases. All 290 procedures were performed by a single practitioner, which included 25 cases following amnioinfusion. Subsequent to this, 15 cases were successful, while 10 cases were not.
After schematization of the CUSUM score, we described the trend line of the quadratic function for each of the three groups; the R2 values of overall CUSUM scores were 0.9892 and 0.9718 for 50% and 30% acceptable ECV failure rates, respectively, and 0.9735 and 0.879 for nullipara subjects, and 0.9925 and 0.9856 for over para 1. The H value, which indicates that the actual failure rate does not differ from the acceptable failure rate, was −3.27 and −1.635 on p1 (acceptable failure rate)=0.5 group and p1 (acceptable failure rate)=0.3 group each. When we assume that the acceptable ECV failure rate was 50% each, the case numbers were 56.94 and 12.82 for nullipara and over para 1, respectively. Furthermore, when we reduce the acceptable ECV failure rate to 30%, the case number was 129.56 for nullipara and 0.75 for over para 1. In other words, in order to obtain a 50% consistent success rate, we need 57 cases of nullipara subjects and 13 cases of over para 1, while for the proficiency of ECV (a consistent 70% success rate), 130 cases of nullipara and one case of over para 1 will be required.
For over para 1 patients, the number of cases needed to achieve an expected ECV success rate of 50% was greater than that required to achieve an expected success rate of 70%. This may be due to the difference between the value of the quadratic function and the real CUSUM score. In fact, when we ascertained the point, which corresponds to the real CUSUM score and H value, we observed that approximately 8 to 10 cases would be necessary for an expected success rate of 50% and 70%, both.
Consequently, after performing 8 to 10 cases of ECV, we could reach a 50% to 70% success rate for over para 1 subjects and after that, achieve a consistent success rate. Otherwise, for nullipara patients, to achieve an expected success rate of 50%, approximately 57 cases are needed, and for a 70% success rate, approximately 130 cases are needed.
There are various advantages of carrying out the present study. Firstly, we have been able to analyze almost 300 cases by a single practitioner, and created a more objective learning curve by using learning curve-cumulative sum (LC-CUSUM) analysis, an established a statistical method for determining cumulative success rate. One study used LC-CUSUM to evaluate an anesthesiology resident; proficiency was evident after 45 cases of intubation, 60 cases of epidural anesthesia, and for cases of endoscopic retrograde cholangiopancreatography, proficiency was noted after approximately 100 cases. LC-CUSUM analysis could therefore be readily applied to a variety of clinical techniques [7]. Secondly, this represents the first study in Korea to evaluate the learning curve for ECV; previously only one other worldwide study had considered this methodology. Although there is some established research regarding learning curves in general surgery, such as the surgical procedure for appendicitis, and in gynecology, including, the learning curve for laparoscopically assisted vaginal hysterectomy or single port laparoscopy, studies pertaining to the learning curve for ECV are very limited, even in other countries. Only one report, published in the Singapore Medical Journal, described the success rate of ECV plateau after the first 20 cases (45% to approximately 60%) and showed that only parity and type of breech had a significant effect on outcome. The success rate was lower for nulliparity and non-flexed breech. This negative effect was the strongest in the first 20 cases and again plateaued after the first 20 cases. The high success rate for multipara subjects with flexed breech was obtained even in the first 20 cases and did not improve with further experience [5]. However, the overall case number was only 80 at that time, and this was a study carried out 20 years ago. And they simply showed the possibility of presence of learning curve and approximate improvement tendency through simple moving average curve but our study was tried to suggest more objective learning curve using special statistic index. Currently however, the number of ECV cases has increased overwhelmingly and there is a clear need for us to create a more systematic and regulated procedure to optimize safety. Finally, by categorizing our patients into three groups and setting two different target values (50% and 70%), we determined objective quantitative data, which will help us reach a proficient level when performing ECV.
And also, there are several other points of our study to consider. First, we included one successful ECV case where a woman who had undergone a previous cesarean section due to fetal distress during labor. This patient had also received successful ECV with her first baby. According to a previous study, complications are uncommon with ECV in women with previous caesarean sections, with a success rate comparable to that of multiparous women [8]. Uterine scars should therefore not be considered as a contraindication and ECV should be offered to women with previous caesarean sections and breech presentation at term [8]. Secondly, our data showed that the more often the AFI was checked and the lower the body mass index and gestational age was, there was a tendency for better success, although this was not statistically significant. Previous studies showed that parity and age were statistically significant factors in relation to success although as the patient becomes older [9], the probability of success for multipara cases will increase. And lastly, we assumed the 50% and 70% success rate by considering several studies. According to Guirguis et al. [10], the success rate for ECV ranged from 30% to 86%, with an mean success rate of 58%; a Cochrane review also showed a mean ECV success rate of 50%. Tan et al. [9] reported that if the estimated probability of successful ECV is less than 32%, then ECV costs more to society and has poorer cost effectiveness for the patient. In contrast, if the probability of successful ECV was greater than 63%, then computer modeling indicated that ECV would be less expensive and have better cost-effectiveness compared to a scheduled cesarean section.
Otherwise, there were some limitations associated with this study, which need to be considered when interpreting our results. Firstly, we included 25 cases of ECV, which were performed after amnioinfusion; this could have affected the success rate. However, as soon as we gather more statistically significantly data regarding ECV after amnioinfusion, we plan to investigate the correlation between amnioinfusion and success rate more precisely. Secondly, we could not create an exact learning curve that incorporates other variables, such as AFI, type of breech, and body mass index. Here, we only categorized according to parity.
However, despite these limitations, our current data are meaningful in that we can now determine the number of cases based on parity to achieve consistent proficiency in the ECV procedure. As the results, for nullipara patients, to achieve an 50% consistent expected success rate to demonstrate, approximately 57 cases are needed, and for a 70% consistent expected success rate to demonstrate, approximately 130 cases are needed. Otherwise, after performing 8 to 10 cases of ECV, we could reach a 50% to 70% success rate for over para 1 subjects and after that, achieve a consistently more proficient success rate.
As noted in a Cochrane review, ECV is the only method that can reduce the rate of cesarean sections in cases where the fetus presents in a breech position, with fewer complications. Even if the physician involved is a beginner, they can experience success with over para 1 patients, and after accumulating relevant experience, they can achieve success with nullipara patients as well. Future research using LC-CUSUM should include several practitioners instead of a single practitioner. By doing this, we may be able to apply standardized learning curve guidelines for ECV in the clinic.
References
1. Kim MY, Park MY, Kim GJ. External cephalic version experiences in Korea. Obstet Gynecol Sci. 2016; 59:85–90. PMID: 27004197.

2. Hofmeyr GJ, Kulier R. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2000; (2):CD000083. PMID: 10796122.

3. Hutton EK, Hannah ME, Ross SJ, Delisle MF, Carson GD, Windrim R, et al. The Early External Cephalic Version (ECV) 2 trial: an international multicentre randomised controlled trial of timing of ECV for breech pregnancies. BJOG. 2011; 118:564–577. PMID: 21291506.

4. Zhang Q, Zhang Q, Guo W, Liu Z, Cheng L, Yue D, et al. The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LC-CUSUM). J Orthop Surg Res. 2014; 9:81. PMID: 25192976.

5. Teoh TG. Effect of learning curve on the outcome of external cephalic version. Singapore Med J. 1997; 38:323–325. PMID: 9364883.
6. Lim SM, Park HS, Jeon CW. Cumulative sum analysis for learning curve for breast mass excision using an ultrasound-guided vacuum-assisted biopsy system. J Breast Dis. 2015; 3:43–47.

7. Seo KY, Sohn YD, Ahn JY, Ahn HC, Cho JH. Evaluation of proficiency in chest compression by learning curve-cumulative sum analysis. J Korean Soc Emerg Med. 2010; 21:293–298.
8. Burgos J, Cobos P, Rodriguez L, Osuna C, Centeno MM, Martinez-Astorquiza T, et al. Is external cephalic version at term contraindicated in previous caesarean section? A prospective comparative cohort study. BJOG. 2014; 121:230–235. PMID: 24245964.

9. Tan JM, Macario A, Carvalho B, Druzin ML, El-Sayed YY. Cost-effectiveness of external cephalic version for term breech presentation. BMC Pregnancy Childbirth. 2010; 10:3. PMID: 20092630.

10. Guirguis GF, Haddad A, Williams SF. External cephalic version. Top Obstet Gynecol. 2016; 36:1–5.
Fig. 1
Overall cumulative sum (CUSUM) score of total patients. (A) Acceptable external cephalic version failure rate of 50%. (B) Acceptable external cephalic version failure rate of 30%.
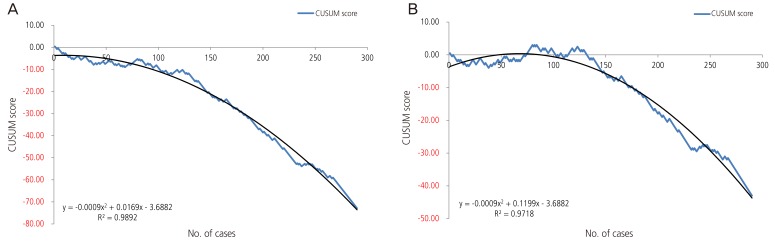
Fig. 2
Cumulative sum (CUSUM) score of nullipara patients. (A) Acceptable external cephalic version failure rate of 50%. (B) Acceptable external cephalic version failure rate of 30%.
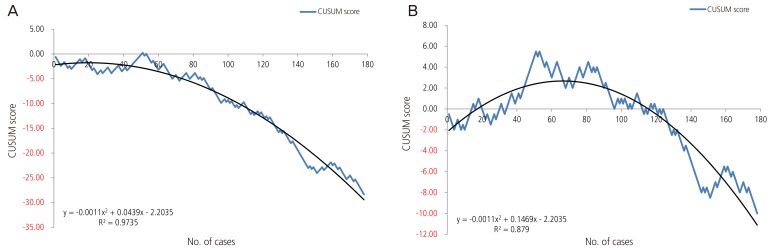
Fig. 3
Cumulative sum (CUSUM) score of over para 1 patients. (A) Acceptable external cephalic version failure rate of 50%. (B) Acceptable external cephalic version failure rate of 30%.
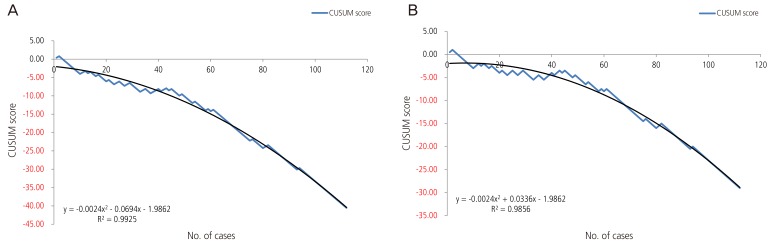
Table 1
Demographic factors of the patients involved in this study
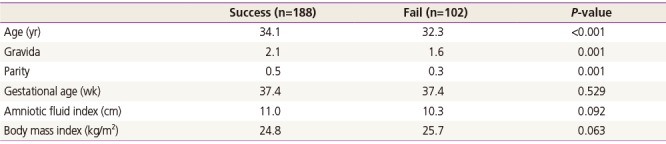
Table 2
Formulas and values involved in plotting the LC-CUSUM curve with an acceptable ECV failure rate of 50%
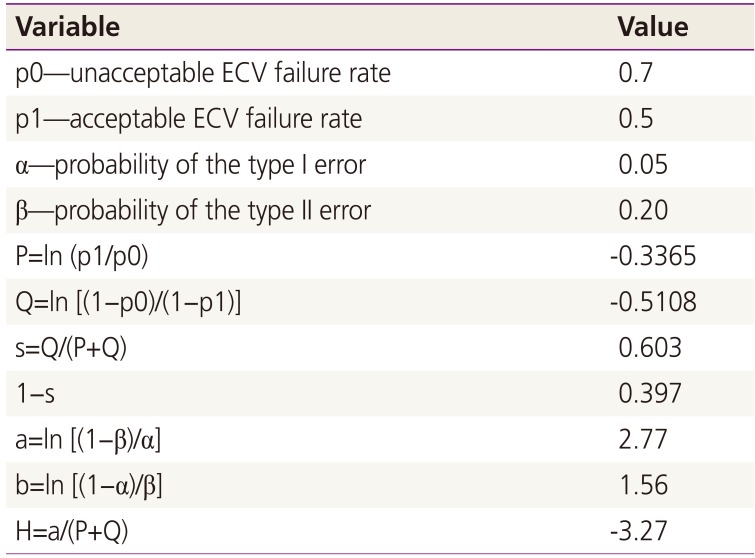
Table 3
Formulas and values involved in plotting the LC-CUSUM curve with an acceptable ECV failure rate of 30%




 PDF
PDF ePub
ePub Citation
Citation Print
Print



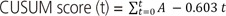
 XML Download
XML Download