Abstract
A single-rod subdermal contraceptive implant is usually located around the insertion site, has been usually known to migrate within less than 2 cm of the insertion site and the true migration over 2 cm has been rarely reported. We report a case of migrated radiopaque subdermal contraceptive implant into lung in a 37-year-old woman. On conducted chest computed tomography, subdermal contraceptive implant was in subsegmental branch in left posterior basal segment of lung. Removal of subdermal contraceptive implant in left posterior basal segment of lung by mini-thoracotomy was performed by a chest surgeon. Complications with insertion and removal of subdermal contraceptive implant are rare in the hands of medical professionals familiar with the techniques and these procedures should only be undertaken by those with relevant training. The migration over 2 cm should not occur if the correct subdermal insertion procedure is followed and carried out by a properly trained individual.
A subdermal contraceptive implant is a single-rod, nonbiodegradable implantable contraceptive that contains the progestin etonogestrel. The contraceptive efficacy of subdermal contraceptive implant was high, with zero pregnancies during treatment with subdermal contraceptive implant, resulting in a Pearl index of 0.0 (95% confidence interval, 0.0 to 0.2) [1]. It has been used widely throughout the world, providing contraceptive protection for up to 3 years when inserted subdermally [234]. Etonogestrel, the active metabolite of desogestrel, is a progestin with a well-established safety and efficacy profile that is also used in a contraceptive etonogestrel/ethinyl estradiol vaginal ring (NuvaRing, Organon USA Inc., Roseland, NJ, USA) [2].
Attention should be paid to careful insertion and removal techniques. Because the rod is nonbiodegradable, subdermal contraceptive implant should be removed after the maximum duration of action or whenever desired. Subdermal contraceptive implant insertion is more complex. Improper insertion of the device may result in migration of the subdermal contraceptive implant over 2 cm [2]. The subdermal contraceptive implant may not be placed in arm at all due to failed insertion. When it occurs, women who received subdermal contraceptive implantation can become pregnant and this may lead to contraception failure and pregnancy [5]. Also, removal of the subdermal contraceptive implant may be very difficult or impossible if the subdermal contraceptive implant is located at unexpected positions. Special procedures, including surgery in the hospital, may be needed to remove the implant, if not properly placed. If the implant is not removed, then the effects of implant will continue for a longer period of time. Other problems related to insertion and removal include pain, irritation, swelling, bruising, scarring, infection, injury to the nerves or blood vessels, and breakage of the implant [6].
We operate a transfer center for patients with difficult implant removal, some implants have been found lying deeply in muscle due to faulty insertion technique or weight gain. Here we report a case of migrated implant into lung.
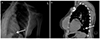
A 37-year-old woman, without medical/surgical history, had a radiopaque subdermal contraceptive implant (Nexplanon, Merck & Co., Whitehouse Station, NJ, USA) inserted in the left upper limb for contraception at a local clinic in 2014. The patients experienced irregular vaginal bleeding for two years after subdermal contraceptive implant and decision was made to remove the device. The subdermal contraceptive implant was not located by digital palpation or by ultrasound scanning of the insertion site. X-ray evaluation of both humerus anterior and posterior was conducted and radiopaque foreign body was not detected. In addition, the long bone upper extremity standing anterior posterior lateral X-ray was performed, and it showed that about 4 cm length rod-like material is seen at the left lower thoracic area (Fig. 1A). Under the impression of foreign body at lower thoracic area, A non-contrast-chest computed tomography (CT) was performed, looking for a possible migration. It revealed that the foreign body was in left lower lobe of the lung (probably in subsegmental pulmonary artery branch in left posterior basal segment) (Fig. 1B). The patient did not experience symptoms including chest discomfort or dyspnea while having the subdermal contraceptive implant. After consultations with cardiology and chest thoracic surgery department, the foreign body removal through intervention was considered initially at the division of cardiology, department of internal medicine. However, the foreign body was present in the lung parenchyme, and the decision was made to remove it through surgery at the department of chest thoracic surgery. The patient was admitted to cardiothoracic department. Under general anesthesia, the patient was positioned in the right decubitus position during a surgery and a 6-cm minithoracotomy has performed at left intercostal space. Left lower lobe of lung was retracted using a long clamp and the site of foreign body was confirmed manually. Bovie cauterization was done to expose the foreign body and enucleation was performed immediately afterward. A 24 Fr-standard chest tube was inserted and the surgery was completed (Fig. 2). The chest tube was removed on postoperative day 2. The patient was discharged from the hospital without complication. And further contraception plan was discussed at the outpatient clinic of the department of obstetrics and gynecology.
The subdermal contraceptive implant is a long-active progestogen-only contraceptive method that contains 68 mg etonogestrel [6]. Significant migrations (>2 cm) are uncommon, and primarily occur caudally looking to the insertion site. Another side effects include deep insertion, fibrous adhesion and broken implant, the prevalence of the side effects are estimated to be 1.1% [7].
In our case, it is estimated that an inadvertent placement of the subdermal contraceptive implant into the basilic vein occurred during the initial procedure. The subdermal contraceptive implant migrated through the upper limb veins, stopping in the pulmonary artery branch in left posterior basal segment, then broke through the pulmonary artery branch and invaded the lung parenchyme. When the subdermal contraceptive implant dislocated in pulmonary artery, intervention could be a method to remove it [8], but in this case thoracotomy was done due to difficult access. In previous case, it was attempted endovascular retrieval by selective catherization of pulmonary artery, using a gooseneck loop snare. After failing many times, hybrid operating room-guided video-assisted thoracoscopic surgery was conducted for removal [9].
Instructions for insertion state that subdermal contraceptive implant should be placed subdermally at the inner side of the upper nondominant arm about 7 cm above the elbow crease in the groove between the biceps and the triceps. The needle of the subdermal contraceptive implant inserter is introduced in the above-mentioned space, directly under the skin. Because it is coated with barium sulphate, it is detactable on X-ray or CT [10]. It is inserted in the subcutaneous plane on the medial aspect of the nondominant arm, 8 to 0 cm proximal to the medial epicondyle under local anesthesia and should be palpable throughout its use. Recommended removal is described in the product information, and is via a small subcutaneous incision at the distal end of the palpable rod, with the rod manually pushed through the incision and grasped with forceps as it appears. Notably, no dissection is required. In the case of the rod not being palpable it is recommended that ultrasound, X-ray, CT or magnetic resonance imaging be used to locate and remove the rod [11]. Previous studies show when the implant is inserted deep into soft tissue, the ultrasound is highly effective in assisting in the removal of impalpable [12]. The majority of impalpable implants were removed under local anesthetic with ultrasound control. The ultrasound-guided blunt dissection, in conjunction with a 22-g spinal needle to lift and stabilize the midpoint of the implant, has been shown to be the most effective technique of removing the implants [13].
Complications with insertion and removal of subdermal contraceptive implant are rare in the hands of medical professionals familiar with the techniques and device, and these procedures should only be undertaken by those with relevant training.
In many cases, when the inserted implant is not palpable, it is located in muscle layer or soft tissue. Imaging studies including X-ray usually detect contraceptive implant placed subdermally in the upper arm but when the implant migrate form their initial implantation, an evaluation on lung and heart is necessary. Measuring serum etonogestrel level should considered among implant users. Also, all women should be informed and consent to disadvantages including device dislocation, irregular vaginal bleeding, unexpected side effects inserting subdermal contraceptive implant.
Figures and Tables
Fig. 1
(A) Long bone upper extremity standing anterior posterior lateral X-ray showing a linear opaque structure (arrow) in pulmonary hemi-right field. (B) Non-contrast chest computed tomography showing that the foreign body (arrow) was in left lower lobe (probably in subsegmental pulmonary artery branch in left posterior basal segment).
References
1. Balogun OR, Olaomo N, Adeniran AS, Fawole AA. Implanon sub-dermal implant: an emerging method of contraception in Ilorin, Nigeria. J Med Biomed Sci. 2014; 3:1–5.
2. Croxatto HB, Urbancsek J, Massai R, Coelingh Bennink H, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999; 14:976–981.
3. Collins DC. Sex hormone receptor binding, progestin selectivity, and the new oral contraceptives. Am J Obstet Gynecol. 1994; 170:1508–1513.
4. Brache V, Faundes A, Alvarez F. Risk-benefit effects of implantable contraceptives in women. Expert Opin Drug Saf. 2003; 2:321–332.
5. Bensouda-Grimaldi L, Jonville-Bera AP, Beau-Salinas F, Llabres S, Autret-Leca E. le reseau des centres regionaux de pharmacovigilance. Insertion problems, removal problems, and contraception failures with Implanon. Gynecol Obstet Fertil. 2005; 33:986–990.
6. Implanon [Internet]. Kenilworth (NJ): Merck & Co.;c2011. cited 2017 Mar 13. Available from: http://www.implanon.com.
7. Edwards JE, Moore A. Implanon: a review of clinical studies. Br J Fam Plann. 1999; 24:3–16.
8. Heudes PM, Querat VL, Darnis E, Defrance C, Douane F, Frampas E. Migration of a contraceptive subcutaneous device into the pulmonary artery: report of a case. Case Rep Womens Health. 2015; 8:6–8.
9. O'Brien A, O'Reilly MK, Sugrue G, Lawler L, Farrelly C. Subdermal contraceptive implant embolism to a pulmonary artery. Ann Thorac Surg. 2015; 99:2254–2255.
10. James P, Trenery J. Ultrasound localisation and removal of non-palpable Implanon implants. Aust N Z J Obstet Gynaecol. 2006; 46:225–228.
11. Diaz S, Pavez M, Miranda P, Robertson DN, Sivin I, Croxatto HB. A five-year clinical trial of levonorgestrel silastic implants (Norplant). Contraception. 1982; 25:447–456.
12. Evans R, Holman R, Lindsay E. Migration of implanon: two case reports. J Fam Plann Reprod Health Care. 2005; 31:71–72.
13. Organon Laboratories. Implanon: summary of product characteristics. place unknown: Organon Laboratories;2003.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download