Abstract
Objective
The aim of this study was to evaluate the association between prenatally diagnosed isolated single umbilical artery (iSUA) and perinatal outcomes.
Methods
We searched Medline, Embase, the Cochrane Library, and KoreaMed from inception to January 2016, with no language or regional restrictions, for cohort and case-control studies reporting on the relationship of iSUA and perinatal outcomes. We assessed the odds ratios (ORs) and 95% confidence intervals (CIs) for the occurrence of small for gestational age, preterm birth, pregnancy-induced hypertension, neonatal intensive care unit admission, and perinatal mortality in fetuses with iSUA compared with those in fetuses with three vessel cord.
Results
Eleven articles totaling 1,731 pregnancies with iSUA met the selection criteria. Studies varied in design, quality, outcome definition, and results. Meta-analysis carried out within predefined groups showed that the presence of an iSUA was associated with small for gestational age (OR, 2.75; 95% CI, 1.97 to 3.83; P<0.00001), preterm birth (OR, 2.10; 95% CI, 1.72 to 2.57; P<0.00001), pregnancy-induced hypertension (OR, 1.62; 95% CI, 1.00 to 2.63; P=0.05), neonatal intensive care unit admission (OR, 2.06; 95% CI, 1.33 to 3.19; P=0.001), and perinatal mortality (OR, 2.29; 95% CI, 1.32 to 3.98; P=0.003).
Conclusion
Pregnancies complicated by iSUA are at increased risk for small for gestational age, preterm birth, pregnancy-induced hypertension, neonatal intensive care unit admission and perinatal mortality. Further, large prospective cohort studies are required to improve the quality of prenatal counseling and the neonatal care for pregnancies with iSUA.
The umbilical cord normally serves as the conduit for two umbilical arteries and one umbilical vein. Single umbilical artery (SUA) is characterized by absence of one of the umbilical arteries and its incidence occurs in 0.5% to 5% of pregnancies [123]. Till now, the mechanism of its development remains uncertain. There are three widely accepted explanations concerning the pathogenesis of an absent umbilical artery: (1) primary agenesis of one umbilical artery; (2) secondary atrophy or atresia of a previously normal developed umbilical artery; and (3) persistence of the original allantoic artery of the body stalk. Embryological considerations, as well as the detection of occluded remnants of a second umbilical artery in some SUA fetuses, suggest that the second theory is the most likely explanation [456].
Fetuses with a SUA are considered at increased risk of chromosomal and structural abnormalities [178910]. Therefore, when SUA is diagnosed at the time of the mid-trimester scan, targeted ultrasound is warranted to rule out underlying pathologic conditions. SUA is defined as an isolated SUA (iSUA), if it occurs with no congenital abnormality in fetal chromosomes or structure [11]; In approximately 65% to 80% of SUAs, it appears to be iSUAs. In pregnancies with an iSUA, variable outcomes of aneuploidy, small for gestational age (SGA), preterm birth, pregnancy-induced hypertension (PIH), neonatal intensive care unit (NICU) admission, and perinatal mortality have been reported when compared with those fetuses and neonates with three vessel cords (TVC) [1112131415161718192021]. However, a controversy exists concerning whether iSUA is associated with adverse perinatal outcomes, with other studies presenting different views.
We carried out a comprehensive systematic review of the literature to evaluate the association of fetuses with prenatally diagnosed iSUA and perinatal outcomes.
This systematic review was protocol driven using widely recommended methods for reviews and evaluation of causal associations. The review was carried out and has been reported according to the PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) and MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines.
We searched Medline, Embase, the Cochrane Library, and KoreaMed, from inception until January 2016 for relevant published articles. “Single umbilical artery,” “two vessel cord,” “two umbilical vessels,” “SUA,” “pregnancy outcome,” and “perinatal outcome” were entered as MeSH (Medical Subject Heading) terms and as keywords. The terms were then connected through Boolean operators and the result was limited to studies reporting only on human subjects and clinical trials. We had no limitations on whether clinical trial was controlled or randomized, on language, or age group. We adapted the Medline search strategy for use in other databases.
Randomized controlled trials, cohort studies and case–control studies were considered eligible if they described iSUA identified by ultrasound before an average gestational age of 24 completed weeks and perinatal outcomes. Studies were excluded if SUA was first diagnosed at birth or if the study was only on twin pregnancies. And we excluded studies with fifty or fewer cases, because of unreliability.
We selected studies for inclusion in a two-stage process. First, two independent reviewers (A and B) screened the titles and abstracts to identify eligible studies; Second, this screening was followed by retrieval and assessment of the full texts of the relevant citations. When the same dataset was reported multiple times, we extracted data from the most recent study with the largest sample size for relevant outcomes. Any disagreements were resolved after discussion with a third reviewer (C).
Data were extracted in duplicate (A and B) with predesigned data collection form. Study characteristics that were summarized on the form included: first author, year of publication, data collection period, country of investigation and number of cases of iSUA and control groups. Quantitative data on the outcome variables included incidence of SGA, PIH, preterm labor, NICU admission and perinatal mortality. Dichotomous data were extracted as 2×2 tables of the association between the iSUA and the perinatal outcome.
We used the Newcastle-Ottawa Scale, which assigns points depending on the quality of patient selection (maximum 4 stars), comparability of the cohort (maximum 2 stars), and outcome assessment (maximum 3 stars) to assess the methodological quality of the included studies. Two independent reviewers (A and B) undertook quality assessment and allocated stars. Studies that scored four stars for selection, two stars for comparability, and three stars for ascertainment of the outcome were regarded to have a low risk of bias. Studies with two or three stars for selection, one for comparability, and two for outcome ascertainment were deemed to have a medium risk of bias. Any study with a score of one for selection or outcome ascertainment, or zero for any of the three domains, was deemed to have a high risk of bias.
Measures of effect used for dichotomous data are the odds ratio (OR). Methods used in this meta-analysis are specific to meta-analysis and include heterogeneity analysis, sensitivity analysis, and evaluation of publication bias. All methods used should allow for the weighting of studies. The concept of weighting reflects the value of the evidence of any particular study. Usually, studies are weighted according to the inverse of their variance. We tested the heterogeneity of results across the studies using the I2 test. If statistical heterogeneity was small (I2<50%) a Mantel-Haenszel fixed-effects model was used. For studies with moderate statistical heterogeneity (I2>50%), we used a Mantel-Haenszel random-effects model. We created funnel plots by plotting the natural logarithm of the ORs against the inverse of the standard error to assess publication and related bias. We statistically checked for the asymmetry of the funnel plot. We identified 11 studies and use software RevMan ver. 5.0 (The Cochrane Collaboration, Oxford, UK) to estimate effects size.
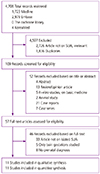
An initial search of 4,706 citations identified from the four databases. Of the 109 eligible articles, we selected 57 studies for detailed assessment. Of these, 46 studies did not meet the inclusion criteria, leaving 11 studies for inclusion in the systematic review. The selection process of the studies and reasons for exclusion are shown in Fig. 1. Table 1 shows the characteristics of the 11 included studies [1112131415161718192021]. The studies comprised 3 case-control studies and 8 cohort studies. These studies reported iSUA cases between 68 and 392, resulting in a total number of cases with an iSUA of 1,731. Quality assessment by the Newcastle-Ottawa Scale showed that all of the included studies had medium risk of bias. One of the 11 studies had low risk of bias for study selection, 10 had medium risk of bias. For comparability of the cohorts, all of the studies had low risk of bias. The risk of bias for outcome assessment was low in 6 studies, medium in 5 studies.
Eleven studies (8 cohort studies and 3 case-control studies) totaling 1,731 fetuses with iSUA reported on the association between SGA and iSUA. All studies had an OR point estimate greater than 1.0 and OR was 2.75, implying that the incidence of SGA in fetuses with iSUA was significantly higher than in fetuses with TVC, and iSUA increased the risk of SGA. Heterogeneity was significant (I2=71%) overall (Fig. 2) [1112131415161718192021].
For preterm birth, 5 studies totaling 127,742 pregnancies examined the association between iSUA and preterm birth. However, these studies did not differentiate between medically indicated and spontaneous preterm deliveries. No heterogeneity was found (chi2=3.09, P=0.66, I2=3%), we chose fixed-effects model to do the meta-analysis. 4 studies had an OR point estimate greater than 1.0. The overall association was OR 2.10 (95% CI, 1.72 to 2.57; P<0.00001). These results suggest that the incidence of preterm birth in fetuses with iSUA was significantly higher than in fetuses with TVC, and iSUA increased the risk of any preterm birth (Fig. 3) [1113171820]
The association between PIH and iSUA were assessed in 7 studies. Significant heterogeneity was found (chi2=17.18, P=0.009, I2=65%) (Fig. 4) [11121317181920], so random-effect model was used. The association between PIH and iSUA were seen in all meta-analyses. The reported ORs in the 7 individual studies ranged from 0.72 to 5.25 and OR was 1.62 (95% CI, 1.00 to 2.63; P=0.05), indicating that the incidence of PIH in fetuses with iSUA was significantly higher than that in fetuses with TVC and iSUA was associated with the occurrence of PIH.
For perinatal mortality, estimates were reported from 5 retrospective cohort studies and one case-control study with 1,176 iSUA cases. No heterogeneity was found (chi2=2.40, P=0.66, I2=0%), we chose fixed-effect model to do the meta-analysis. This meta-analysis showed that perinatal mortality is significantly associated with iSUA (OR, 2.29; 95% CI, 1.32 to 3.98; P=0.003) (Fig. 6) [131415161718].
This study performed a meta-analysis of 11 studies that considered the relationship between prenatally diagnosed iSUA and perinatal outcomes, including SGA, preterm birth, PIH, and perinatal mortality. There has been controversy regarding the association between iSUA and fetal growth. Tulek et al. [11] analyzed birth weight and found that SGA was three-fold as likely in pregnancies with iSUA compared to pregnancies with TVC. Other recent studies also reported similar results [1317181920]. However, Bombrys et al. [14] found that iSUA was not associated with a higher rate of SGA in comparison with newborns with TVC (13.7% vs. 13.9%, P=0.93). Horton et al. [16] reported that even though the ponderal index, which is a more accurate measure of neonatal slenderness, was significantly lower in neonates with iSUA compared with TVC neonates (24.2±1.1 vs. 26.1±1.3 g/cm3, P=0.001), there was no significant difference in rates of SGA (17.6% vs. 8.8%, P=0.06).
The association between preterm birth and iSUA is also controversial. Some studies have shown a higher risk of preterm birth in cases with iSUA [1320] and others have demonstrated no association [11]. Hua et al. [17] did not find a higher rate of preterm birth when comparing newborns with iSUA with those with normal umbilical cord. Murphy-Kaulbeck et al. [22] demonstrated that rates of prematurity were significantly higher for less than 37 weeks of gestation (OR, 2.48; 95% CI, 1.91 to 3.23).
The relationship between iSUA and perinatal mortality also remains controversial. In the study conducted by Bombrys et al. [14], the finding of iSUA was not associated with increased perinatal morbidity or mortality. Burshtein et al. [23] reported iSUA as an independent risk factor for perinatal mortality (OR, 7.78; 95% CI, 4.68 to 12.95).
It has been hypothesized that the high incidence of SGA, NICU admission, and perinatal mortality in fetuses with iSUA may occur secondary to a relatively high prevalence of preterm birth [1112131415]. But it is not clear why the incidence of gestational hypertension and preeclampsia is higher for fetuses that have a single umbilical artery but appear to be structurally normal. One hypothesis is that fetuses with single umbilical arteries are at greater risk of anomalous cord development, as part of a wider pathological process affecting the placenta and perfusion rather than inadequate fetoplacental blood flow through the remaining umbilical artery [15]. Haugen et al. [24] reported further evidence in support of this hypothesis by showing that SUA displayed markedly higher outflow levels of prostacyclin, endothelium-derived relaxing factor. Moreover, the vasoactive intestinal peptide that induces dose-dependent relaxation is able to increase the prostacyclin formation in the two umbilical arteries rather than in the SUA [242526].
The first systematic review and meta-analysis on the association between the presence of an iSUA and perinatal outcomes was published in 2013, and reported that there was no statistically significant difference in perinatal mortality between fetuses with an iSUA and TVC [27]. As stated in that meta-analysis, this non-significant result might be due to the limited sample size; the study included only 7 eligible papers covering 928 cases with iSUA. In contrast, our meta-analysis included 11 eligible studies covering 1,731 pregnancies with iSUA.
In our meta-analysis, there were statistically significant differences in the incidence of SGA, preterm birth, PIH, and perinatal mortality between iSUA and TVC fetuses, implying that the incidence of these complications was correlated with iSUA, and that iSUA may increase the risk of these complications.
This meta-analysis provides the best currently available evidence of an association between iSUA and perinatal outcomes. This review provides much needed information about iSUA and perinatal outcomes and will help in the counselling of women with a fetus with iSUA. To our knowledge, this review is the most comprehensive meta-analysis evaluating the association of iSUA and perinatal outcomes. We performed an extensive literature search without language restrictions, thereby increasing the likelihood of retrieving all relevant studies.
This review has several limitations. The main weakness of these studies was the retrospective design and the fact that most of the outcomes were explored by a limited number of studies, despite our efforts to identify all relevant literature. Although the quality assessment of the studies was generally high, the heterogeneity was significant for all outcomes analyzed. Since large or extreme heterogeneity may lead to a misleading conclusion, the degree of heterogeneity is one of the main concerns in meta-analysis. In this study, significant heterogeneity was present in the overall analysis.
Our meta-analysis has provided a systematic review of the association between the presence of an iSUA and various perinatal outcomes. Our results suggest that iSUA is associated with a significant increase in adverse perinatal outcomes such as SGA, preterm birth, PIH, admission to the NICU, and perinatal mortality.
Figures and Tables
Fig. 2
Forest plot of odds ratio with 95% confidence interval (CI) for small for gestational age in fetuses with isolated single umbilical artery (SUA) compared with fetuses with three vessel cord (TVC). M-H, Mantel-Haenszel test.

Fig. 3
Forest plot of odds ratio with 95% confidence interval (CI) for preterm birth in fetuses with isolated single umbilical artery (SUA) compared with fetuses with three vessel cord (TVC). M-H, Mantel-Haenszel test.

Fig. 4
Forest plot of odds ratio with 95% confidence interval (CI) for pregnancy-induced hypertension in fetuses with isolated single umbilical artery (SUA) compared with fetuses with three vessel cord (TVC). M-H, Mantel-Haenszel test.

Fig. 5
Forest plot of odds ratio with 95% confidence interval (CI) for neonatal intensive care unit admission in fetuses with isolated single umbilical artery (SUA) compared with fetuses with three vessel cord (TVC). M-H, Mantel-Haenszel test.

Fig. 6
Forest plot of odds ratio with 95% confidence interval (CI) for perinatal mortality in fetuses with isolated single umbilical artery (SUA) compared with fetuses with three vessel cord (TVC). M-H, Mantel-Haenszel test.

Table 1
Characteristics of studies in the meta-analysis

| Author | Year | Country | Study design | Follow up | SUA | TVC |
|---|---|---|---|---|---|---|
| Gornall [15] | 2003 | UK | Cohort retrospective | 1995–2001 | 107 | 214 |
| Predanic [21] | 2005 | USA | Case control | 1999–2004 | 84 | 84 |
| Bombrys [14] | 2008 | USA | Cohort retrospective | 2000–2006 | 255 | 289 |
| Horton [16] | 2010 | USA | Case control | 2000–2008 | 68 | 68 |
| Hua [17] | 2010 | USA | Cohort retrospective | 1990–2007 | 392 | 63,655 |
| Khalil [18] | 2013 | Saudi Arabia | Cohort retrospective | 2004–2012 | 159 | 35,026 |
| Mailath-Pokorny [19] | 2015 | Austria | Cohort retrospective | 2004–2011 | 136 | 500 |
| Ashwal [12] | 2014 | Israel | Case control | 2008–2012 | 91 | 182 |
| Battarbee [13] | 2015 | USA | Cohort retrospective | 2007–2014 | 219 | 219 |
| Naveiro-Fuentes [20] | 2016 | Spain | Cohort retrospective | 2007–2014 | 127 | 27,752 |
| Tülek [11] | 2015 | Turkey | Cohort retrospective | 2006–2013 | 93 | 100 |
References
1. Doornebal N, de Vries TW, Bos AF, de Vries NK. Screening infants with an isolated single umbilical artery for renal anomalies: nonsense? Early Hum Dev. 2007; 83:567–570.
2. Granese R, Coco C, Jeanty P. The value of single umbilical artery in the prediction of fetal aneuploidy: findings in 12,672 pregnant women. Ultrasound Q. 2007; 23:117–121.
3. Parilla BV, Tamura RK, MacGregor SN, Geibel LJ, Sabbagha RE. The clinical significance of a single umbilical artery as an isolated finding on prenatal ultrasound. Obstet Gynecol. 1995; 85:570–572.
4. Heifetz SA. Single umbilical artery: a statistical analysis of 237 autopsy cases and review of the literature. Perspect Pediatr Pathol. 1984; 8:345–378.
5. Monie IW. Genesis of single umbilical artery. Am J Obstet Gynecol. 1970; 108:400–405.
6. Nyberg DA, Mahony BS, Luthy D, Kapur R. Single umbilical artery: prenatal detection of concurrent anomalies. J Ultrasound Med. 1991; 10:247–253.
7. Chetty-John S, Zhang J, Chen Z, Albert P, Sun L, Klebanoff M, et al. Long-term physical and neurologic development in newborn infants with isolated single umbilical artery. Am J Obstet Gynecol. 2010; 203:368.e1–368.e7.
8. Chow JS, Benson CB, Doubilet PM. Frequency and nature of structural anomalies in fetuses with single umbilical arteries. J Ultrasound Med. 1998; 17:765–768.
9. Dagklis T, Defigueiredo D, Staboulidou I, Casagrandi D, Nicolaides KH. Isolated single umbilical artery and fetal karyotype. Ultrasound Obstet Gynecol. 2010; 36:291–295.
10. Thummala MR, Raju TN, Langenberg P. Isolated single umbilical artery anomaly and the risk for congenital malformations: a meta-analysis. J Pediatr Surg. 1998; 33:580–585.
11. Tulek F, Kahraman A, Taskın S, Ozkavukcu E, Soylemez F. Determination of risk factors and perinatal outcomes of singleton pregnancies complicated by isolated single umbilical artery in Turkish population. J Turk Ger Gynecol Assoc. 2015; 16:21–24.
12. Ashwal E, Melamed N, Hiersch L, Edel S, Bardin R, Wiznitzer A, et al. The impact of isolated single umbilical artery on labor and delivery outcome. Prenat Diagn. 2014; 34:581–585.
13. Battarbee AN, Palatnik A, Ernst LM, Grobman WA. Association of isolated single umbilical artery with small for gestational age and preterm birth. Obstet Gynecol. 2015; 126:760–764.
14. Bombrys AE, Neiger R, Hawkins S, Sonek J, Croom C, McKenna D, et al. Pregnancy outcome in isolated single umbilical artery. Am J Perinatol. 2008; 25:239–242.
15. Gornall AS, Kurinczuk JJ, Konje JC. Antenatal detection of a single umbilical artery: does it matter? Prenat Diagn. 2003; 23:117–123.
16. Horton AL, Barroilhet L, Wolfe HM. Perinatal outcomes in isolated single umbilical artery. Am J Perinatol. 2010; 27:321–324.
17. Hua M, Odibo AO, Macones GA, Roehl KA, Crane JP, Cahill AG. Single umbilical artery and its associated findings. Obstet Gynecol. 2010; 115:930–934.
18. Khalil MI, Sagr ER, Elrifaei RM, Abdelbasit OB, Halouly TA. Outcomes of an isolated single umbilical artery in singleton pregnancy: a large study from the Middle East and Gulf region. Eur J Obstet Gynecol Reprod Biol. 2013; 171:277–280.
19. Mailath-Pokorny M, Worda K, Schmid M, Polterauer S, Bettelheim D. Isolated single umbilical artery: evaluating the risk of adverse pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2015; 184:80–83.
20. Naveiro-Fuentes M, Carrillo-Badillo MP, Malde-Conde J, Gallo-Vallejo JL, Puertas-Prieto A. Perinatal outcomes in singleton pregnancies with a single umbilical artery. J Matern Fetal Neonatal Med. 2016; 29:1562–1565.
21. Predanic M, Perni SC, Friedman A, Chervenak FA, Chasen ST. Fetal growth assessment and neonatal birth weight in fetuses with an isolated single umbilical artery. Obstet Gynecol. 2005; 105:1093–1097.
22. Murphy-Kaulbeck L, Dodds L, Joseph KS, Van den Hof M. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010; 116:843–850.
23. Burshtein S, Levy A, Holcberg G, Zlotnik A, Sheiner E. Is single umbilical artery an independent risk factor for perinatal mortality? Arch Gynecol Obstet. 2011; 283:191–194.
24. Haugen G, Stray-Pedersen S, Bjoro K. Vasoactive intestinal peptide and prostanoid production in normal and single umbilical artery cords in normotensive and hypertensive pregnancies. Clin Exp Hypertens B. 1990; 9:69–80.
25. Maigaard S, Forman A, Andersson KE. Digoxin inhibition of relaxation induced by prostacyclin and vasoactive intestinal polypeptide in small human placental arteries. Placenta. 1985; 6:435–443.
26. Hansen V, Maigaard S, Allen J, Forman A. Effects of vasoactive intestinal polypeptide and substance P on human intramyometrial arteries and stem villous arteries in term pregnancy. Placenta. 1988; 9:501–506.
27. Voskamp BJ, Fleurke-Rozema H, Oude-Rengerink K, Snijders RJ, Bilardo CM, Mol BW, et al. Relationship of isolated single umbilical artery to fetal growth, aneuploidy and perinatal mortality: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013; 42:622–628.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download