Abstract
Objective
To describe the endometrial pathologic lesions in premenopausal breast cancer patients with a history of tamoxifen (TMX) use.
Methods
We retrospectively reviewed the medical records of 120 premenopausal breast cancer patients with a history of TMX use that had undergone a gynecological examination.
Results
Among 120 patients, 44.2% (n=53) were asymptomatic with an endometrial thickness ≥5 mm, as assessed by transvaginal ultrasonography. Of the patients that reported abnormal uterine bleeding, 5% (n=6) had an endometrial thickness <5 mm and 20% (n=24) had an endometrial thickness ≥5 mm by transvaginal ultrasonography. The final group of patients were asymptomatic, but showed an abnormal endometrial lesion, such as an endometrial polyp, by transvaginal ultrasonography (30.8%, n=37). Of the 56 benign lesions that were histologically reviewed, 50 (41.7%) were endometrial polyps, 3 (2.5%) were submucosal myomas, 2 (1.7%) were endometrial hyperplasias, and 1 (0.8%) was chronic endometritis. There were 64 (53.3%) other non-pathologic conditions, including secreting, proliferative, and atrophic endometrium, or in some cases, there was insufficient material for diagnosis. In our data, only one case was reported as a complex hyperplasia without atypia arising from an endometrial polyp, and one patient was diagnosed with endometrioid adenocarcinoma.
Conclusion
For premenopausal breast cancer patients with a history of TMX use, the majority of the patients were asymptomatic, and endometrial polyps were the most common endometrial pathology observed. Therefore, we believe that endometrial assessment before starting TMX treatment, and regular endometrial screening throughout TMX treatment, are reasonable suggestions for premenopausal breast cancer patients.
The incidence of breast cancer has risen continuously over the past 10 years, and it is the second most common cancer in Korean women. More than half of the newly diagnosed breast cancer patients are diagnosed as stage I or II. In Korea, the median age at breast cancer diagnosis is 51 years, and the highest number of newly diagnosed breast cancer patients are between the ages of 40 to 49 years [1], which are younger than those reported globally.
Tamoxifen (TMX), a selective estrogen receptor (ER) modulator, is used as an adjuvant endocrine therapy for ER-positive breast cancer patients, and in these patients, it has been effective in reducing the risk of recurrence and mortality [23]. Recent data have shown that continuing TMX therapy for 10 years after diagnosis in ER-positive breast cancer patients results in a greater reduction in recurrence and mortality than those using TMX for just 5 years [45].
However, other studies have shown that endometrial cancer is significantly associated with postmenopausal breast cancer patients treated with TMX, and the risk increases with the increased duration of TMX treatment [678]. For instance, Saccardi et al. [9] showed that, although the risk ratio (RR) for endometrial cancer is low in women receiving TMX therapy who are <50 years old (RR, 1.19), it is significantly increased in those >50 years old (RR, 3.32). Moreover, the endometrial polyps recovered from postmenopausal TMX-treated patients show a high rate of malignant transformation with a high grade of malignancy; however, vaginal bleeding is only associated with 50.0% of these cases [10111213]. Interestingly, these studies found no correlation between malignant polyp formation and polyp size or treatment duration [101213]. Furthermore, studies have shown that, among all patients with endometrial polyps, the prevalence rate of premalignant and malignant endometrial polyps is low (3.57%); however, postmenopausal women have a higher prevalence of malignancy than premenopausal women (4.67% vs. 1.95%) [14]. Similarly, other studies have reported malignant transformation rates in 3.0% to 10.7% of endometrial polyps recovered from postmenopausal TMX-treated breast cancer patients, which is higher than that reported in healthy controls [15].
To date, there are limited studies that have evaluated the risk of premalignant and malignant endometrial polyp formation by hysteroscopy specifically in premenopausal TMX-treated patients. Moreover, both premenopausal and postmenopausal women show increased endometrial polyp formation secondary to TMX use [16], and it has been suggested that endometrial polyps form an intermediate stage between benign endometrial lesions and endometrial malignancy [17]. Considering the high proportion of young, premenopausal breast cancer patients in Korea, it is critical to define the characteristics of premenopausal breast cancer patients who have received, or are currently receiving, TMX treatment, and to identify any potential endometrial effects that may be due to this treatment. Thus, the aim of the present study was to describe the prevalence of premalignant and malignant endometrial polyps in premenopausal TMX-treated breast cancer patients, and to evaluate the risk factors associated with them.
A total of 120 premenopausal breast cancer patients who had used, or were using, TMX, with ages ranging from 27 to 50 years, were included in this study. Patient menopausal status was based on menstrual history and serum hormone levels. Premenopausal patients had regular menstrual cycles or menstruation within 3 months before the start day of chemotherapy, and serum FSH levels <40 mIU/mL at the time of endometrial evaluation. Postmenopausal patients had amenorrhea for more than 12 months. We defined chemotherapy-induced amenorrhea (CIA) for premenopausal patients at the time of diagnosis as 6 consecutive months without a menstrual period. Transvaginal ultrasonography (TV-USG) was performed regardless of the menstrual cycle day. Our protocol was approved by the institutional review board. All patients had undergone surgery for breast cancer and had post-operative treatment, including TMX, gonadotropin-releasing hormone agonist, chemotherapy, and radiotherapy.
All women had undergone TV-USG and diagnostic or surgical hysteroscopy for the diagnosis and treatment of diverse uterine conditions at the Seoul National University Hospital from December 2014 through March 2016. Each patient met one of the following inclusion criteria: (1) asymptomatic, with endometrial thickness ≥5 mm; (2) abnormal uterine bleeding (AUB), as reported by patient, with endometrial thickness <5 mm; (3) AUB, as reported by patient, with endometrial thickness ≥5 mm; or (4) asymptomatic, with abnormal endometrial lesions, including endometrial polyp, identified by TV-USG.
Diagnostic hysteroscopy was performed in the outpatient clinic by gynecologists using a 5-mm hysteroscope (2140, Richard Wolf Medical Instruments Corp., Vernon Hills, IL, USA). For distension of the uterine cavity, a normal saline infusion was used. If an endometrial lesion was identified, and if it was determined to be necessary by the gynecologist performing the hysteroscopy, an immediate outpatient hysteroscopic polypectomy or procedure was performed.
In the case of a large polyp, severe endometrial stenosis, or when the patient was unable to tolerate the procedure, a deferred inpatient surgical hysteroscopy was performed under monitored an aesthesia care. A 10-mm resectoscope (Karl Storz Endoskope, Tuttlingen, Germany) was used for the surgical procedure, and urosol was used as the distension medium. In each patient, evaluation of the endocervical canal, endometrial surface, tubal ostia, endometrial polyps, myomas and synechia was performed. In all cases, the resected lesion was sent for histological examination.
Clinicopathologic data, ultrasound and hysteroscopic findings were collected from medical records retrospectively. The following variables were included: age, body mass index (BMI), TMX treatment duration, stage of breast cancer, breast tumour size and hormone status, history of chemotherapy treatment, history of CIA, presence of menstruation, endometrial thickness, and presence of AUB. Statistical analysis was performed with IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). Age, BMI, duration of TMX treatment, and endometrial thickness were compared using Student's t-test. Breast cancer stage, breast tumor size, presence of menstruation, and presence of AUB were compared using the chi-square test. P-values <0.05 were considered significant.
Demographic and clinicopathologic characteristics of 120 premenopausal patients with a history of breast cancer and subsequent TMX therapy are shown in Table 1. The mean age of the patients was 44 years (range, 27 to 50 years). The mean BMI was 22.4±5.5 kg/m2, and the median duration of TMX treatment was 3 years (range, 0 to 10 years). Most of the patients were diagnosed with breast cancer at an early stage: 14.2% (n=17), stage 0; 44.2% (n=53), stage I; 32.5% (n=39), stage II; and 9.2% (n=11), stage III. As expected, almost all of the patients (96.7%, n=116) were ER-positive, and 83.3% (n=100) were progesterone receptor-positive, whereas 64.2% (n=77) were human epidermal growth factor receptor 2-positive. Moreover, 47.5% (n=57) of the patients received cytotoxic chemotherapy before or after breast surgery, and of those, 96.5% (55/57) experienced CIA.
Regarding their uterine health, 25.0% (n=30) of the patients presented with AUB, and the mean patient endometrial thickness, as evaluated by TV-USG, was 11 mm (range, 3 to 30 mm). For all of the patients, the indication of hysteroscopy and histologic diagnosis of resected lesions are summarized in Table 2. Among 120 patients, 44.2% (n=53) were asymptomatic with an endometrial thickness ≥5 mm, as assessed by TV-USG. Of the patients that reported AUB symptoms, TV-USG showed that 5% (n=6) had an endometrial thickness <5 mm, whereas 20% (n=24) showed an endometrial thickness ≥5 mm. Finally, TV-USG revealed that 30.8% (n=37) of asymptomatic patients actually had an abnormal endometrial lesion, such as a polyp.
A total of 56 benign lesions were histologically reviewed. Of those, 50 (41.7%) were endometrial polyps, 3 (2.5%) were submucosal myomas, 2 (1.7%) were endometrial hyperplasias, and 1 (0.8%) was chronic endometritis. In addition, there were 64 (53.3%) other uterine conditions that were determined to be non-pathologic after further examination, including secreting endometrium, proliferative endometrium, atrophic endometrium, or in some cases, there was insufficient material for diagnosis.
Of all the histologically reviewed lesions, two cases were hyperplasias, including a simple hyperplasia and complex hyperplasia without atypia arising from an endometrial polyp, and only one patient was diagnosed with endometrioid adenocarcinoma. This patient was 50 years old with AUB, took TMX for 9 years, and had an endometrial thickness of 9 mm, which are typical risk factors of endometrial cancer.
Finally, we also determined whether there were any risk factors associated with the presence of endometrial polyps in TMX-treated patients (Table 3). There were no significant correlations between age, BMI, duration of TMX use, breast cancer stage, breast tumour size, menstruation, or endometrial thickness and the histological diagnosis of endometrial polyps. However, the presence of AUB was significantly higher in patients without endometrial polyps than in those with endometrial polyps (P<0.001). Moreover, multiple regression analysis showed that the presence of AUB was the only factor associated with a lower risk of endometrial polyps, with a RR of 0.19 (95% confidence interval, 0.064 to 0.587) (Table 4).
Previous studies have shown that, with standard dosages, TMX may be associated with endometrial proliferation, hyperplasia, polyp formation, invasive carcinoma, and uterine sarcoma [181920]. Endometrial polyps are a common pathology and have an increased malignant transformation rate in postmenopausal TMX-treated breast cancer patients; however, limited studies have investigated the malignant potential of endometrial polyps by hysteroscopy in premenopausal TMX-treated, breast cancer patients. In this study, we evaluated the prevalence of premalignant and malignant endometrial polyps, and the potential risk factors associated with these polyps, in premenopausal, TMX-treated, breast cancer patients. We found that the prevalence rate of endometrial polyps in premenopausal patient with TMX use was 41.7%, and that the majority of these patients were asymptomatic. One patient was identified with a complex hyperplasia without atypia arising from an endometrial polyp, and one patient was diagnosed with endometrioid adenocarcinoma. Moreover, we found that the presence of AUB was significantly higher in patients with non-polyp endometrial lesions than in those with endometrial polyps.
Despite the widespread use of TMX, there are no universally accepted international guidelines for its use. According to suggestions by the American Congress of Obstetricians and Gynecologists (ACOG), premenopausal patients with a history of TMX use have no known increased risk for endometrial cancer and require no further monitoring beyond routine gynecologic exams [16]. However, resections of endometrial polyps that are ultrasonographically diagnosed in postmenopausal, TMX-treated patients are recommended due to their high rate of malignancy. Because of their potential for malignant transformation, even though it is reduced compared with that of postmenopausal patients, we believe the removal of endometrial polyps should also be considered in premenopausal, TMX-treated, breast cancer patients.
There are a number of limitations to this study. First, because this is a retrospective study, we have no endometrial evaluation data before starting TMX treatment, except for the patients who had previously undergone a polypectomy for endometrial polyps. Most of the asymptomatic patients treated with TMX underwent yearly TV-USG examinations, often with subsequent hysteroscopic evaluation. Secondly, this study has limitations stemming from its small sample size, and there were no cases of endometrial cancer associated with endometrial polyps. Thus, a large-scale study assessing premalignant and malignant endometrial polyps in premenopausal breast cancer patients with a history of TMX use is needed.
In conclusion, in premenopausal breast cancer patients with a history of TMX use, polyps were the most common endometrial pathology observed, and most patients showed no associated symptoms. Therefore, based on our observations, we recommend endometrial assessment before starting TMX treatment and endometrial screening every 12 months during TMX treatment as a reasonable option for young, premenopausal breast cancer patients. This recommendation is particularly relevant in Korea, where the median age at breast cancer diagnosis is younger than in other countries. Furthermore, Korea's medical system provides low medical expenses for patients, and regular endometrial screening for the early detection and resection of premalignant endometrial lesions could greatly reduce long-term health care costs. Clearly, more research is needed on the cost effectiveness of endometrial screening in premenopausal breast cancer patients in Korea.
Figures and Tables
Table 1
Clinicopathologic and demographic characteristics of patients (n=120)

Values are presented as median (range), mean±standard deviation, or number (%).
BMI, body mass index; TMX, tamoxifen; ER, oestrogen receptor; PR, progesterone receptor; HER2, humanepidermal growth factor receptor 2; CTx, chemotherapy; CIA, chemotherapy-induced amenorrhea; AUB, abnormal uterine bleeding.
Table 2
Indication to hysteroscopy and histologic results of patients
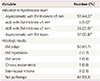
Table 3
Clinical characteristics of patients with or without endometrial polyps

References
1. Kim Z, Min SY, Yoon CS, Jung KW, Ko BS, Kang E, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer. 2015; 18:103–111.
2. Figg WD 2nd, Cook K, Clarke R. Aromatase inhibitor plus ovarian suppression as adjuvant therapy in premenopausal women with breast cancer. Cancer Biol Ther. 2014; 15:1586–1587.
3. Le Donne M, Alibrandi A, Ciancimino L, Azzerboni A, Chiofalo B, Triolo O. Endometrial pathology in breast cancer patients: Effect of different treatments on ultrasonographic, hysteroscopic and histological findings. Oncol Lett. 2013; 5:1305–1310.
4. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013; 381:805–816.
5. Goss PE. Preventing relapse beyond 5 years: the MA.17 extended adjuvant trial. Semin Oncol. 2006; 33:2 Suppl 7. S8–S12.
6. Sismondi P, Biglia N, Volpi E, Giai M, de Grandis T. Tamoxifen and endometrial cancer. Ann N Y Acad Sci. 1994; 734:310–321.
7. Cohen I, Altaras MM, Shapira J, Tepper R, Rosen DJ, Cordoba M, et al. Time-dependent effect of tamoxifen therapy on endometrial pathology in asymptomatic postmenopausal breast cancer patients. Int J Gynecol Pathol. 1996; 15:152–157.
8. Cheng WF, Lin HH, Torng PL, Huang SC. Comparison of endometrial changes among symptomatic tamoxifen-treated and nontreated premenopausal and postmenopausal breast cancer patients. Gynecol Oncol. 1997; 66:233–237.
9. Saccardi C, Gizzo S, Patrelli TS, Ancona E, Anis O, Di Gangi S, et al. Endometrial surveillance in tamoxifen users: role, timing and accuracy of hysteroscopic investigation. Observational longitudinal cohort study. Endocr Relat Cancer. 2013; 20:455–462.
10. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998; 91:40–44.
11. Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000; 78:181–186.
12. Ramondetta LM, Sherwood JB, Dunton CJ, Palazzo JP. Endometrial cancer in polyps associated with tamoxifen use. Am J Obstet Gynecol. 1999; 180(2 Pt 1):340–341.
13. Cohen I, Bernheim J, Azaria R, Tepper R, Sharony R, Beyth Y. Malignant endometrial polyps in postmenopausal breast cancer tamoxifen-treated patients. Gynecol Oncol. 1999; 75:136–141.
14. Costa-Paiva L, Godoy CE Jr, Antunes A Jr, Caseiro JD, Arthuso M, Pinto-Neto AM. Risk of malignancy in endometrial polyps in premenopausal and postmenopausal women according to clinicopathologic characteristics. Menopause. 2011; 18:1278–1282.
15. Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004; 94:256–266.
16. Committee Opinion No. 601: Tamoxifen and uterine cancer. Obstet Gynecol. 2014; 123:1394–1397.
17. Ismail SM. Pathology of endometrium treated with tamoxifen. J Clin Pathol. 1994; 47:827–833.
18. Hoogendoorn WE, Hollema H, van Boven HH, Bergman E, de Leeuw-Mantel G, Platteel I, et al. Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res Treat. 2008; 112:99–108.
19. Le Donne M, Lentini M, De Meo L, Benedetto V, Mesiti M. Uterine pathologies in patients undergoing tamoxifen therapy for breast cancer: ultrasonographic, hysteroscopic and histological findings. Eur J Gynaecol Oncol. 2005; 26:623–626.
20. Buijs C, Willemse PH, de Vries EG, Ten Hoor KA, Boezen HM, Hollema H, et al. Effect of tamoxifen on the endometrium and the menstrual cycle of premenopausal breast cancer patients. Int J Gynecol Cancer. 2009; 19:677–681.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download