Abstract
Extra pelvic endometriosis is considered to be rare. This paper reports a case of catamenial hemoptysis accompanied by subcutaneous endometriosis in 26-year-old woman. A computed tomography scan of the chest revealed a focal ground-glass opacity lesion in the posterior segment of the right upper lobe. Histopathology confirmed the diagnosis of endometriosis of right lung and concurrent subcutaneous endometriosis. She was treated with surgical resection of the endometriosis lesions on two different sites and perioperative gonadotropin-releasing hormone agonist therapy. The 6-month follow-up after combination treatment showed no recurrence. Though long-term follow-up result is needed, aggressive treatment using combination treatment (surgery and perioperative medication) should be considered for symptomatic extra pelvic endometriosis.
Endometriosis is a common disease, affecting 10% to 15% of reproductive aged women. It can be characterized as pelvic or extra pelvic, depending on the site of identification. Extra pelvic endometriosis refers to an existence of endometrial implants in various site, such as gastrointestinal tract, genitourinary tract, cutaneous tissue and lung. Extra pelvic endometriosis is a rare disease entity, furthermore coexistence of two different types of extra pelvic endometriosis is extremely rare. Catamenial hemoptysis (CH) is a clinical presentation of thoracic endometriosis syndrome (TES). There is no established guideline for managing TES; however, surgical or hormonal therapy is generally used to prevent symptom recurrence. We report a patient with CH accompanied by subcutaneous endometriosis who was treated with thoracoscopic surgery and perioperative gonadotropin-releasing hormone (GnRH) agonist therapy.
A 26-year-old woman, gravida 1, para 1, was referred to an outpatient clinic for recurrent hemoptysis associated with her menstrual cycle and a palpable nodule near a previous cesarean scar site. She had a regular period and mild dysmenorrhea since her menarche at 12 years old. She complained of bloody sputum on the first day of her menstrual cycle which repeated for 4 months. She had no other respiratory symptoms such as a fever, chill, or chest discomfort. She never smoked, and her medical history was unremarkable. There was no other history of pelvic surgery or endometrial manipulation except cesarean section.
On physical examination, there was 1-cm painless nodule located 2 cm above the cesarean scar. The lungs were clear on auscultation. Complete blood count results showed mild anemia (hemoglobin concentration 9.9 g/dL) and a mild increase in the platelet count (373 K/µL). The other serum profiles were normal, including blood chemistry, electrolytes, and liver and renal function test. The CA 125 level was within normal range (22 U/mL).
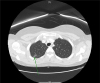
Transvaginal ultrasonography results showed a small intramural myoma and normal ovaries. Results of the preoperative chest radiograph and electrocardiogram were normal. Computed tomography (CT) scan of the chest demonstrated a focal ground-glass opacity lesion in the posterior segment of the right upper lobe, which was consistent with pulmonary endometriosis (catamenial syndrome) (Fig. 1). Magnetic resonance imaging scan of the pelvis demonstrated focal adenomyosis of the left side of the uterus and no endometriosis cyst in both adnexa.
Since pulmonary endometriosis was tentatively diagnosed, we planned to surgically resect the lesion. Preoperatively, GnRH agonist therapy (leuprolide acetate, 3.75 mg) was administered as a subcutaneous injection to suppress ovarian estrogen release. Video-assisted thoracoscopic surgery (VATS) wedge resection was performed in the right upper lobe. The frozen section showed that the specimen was an alveolar hemorrhage. Resection of the subcutaneous nodule was performed concurrently. The patient was discharged without any complications 2 days postoperatively.
On histopathology, endometrial tissue, composed of glands and stroma, was found within the lung parenchyma (Fig. 2A). Intra-alveolar hemorrhage and many hemosiderin-laden macrophages were also identified. Results of the CD10 immunohistochemical staining (1:200, clone: 56C6, Leica Microsystems, Bensheim, Germany) showed endometrial stromal cells (Fig. 2B). The subcutaneous mass was confirmed to be endometriosis.
After 2 weeks, we started GnRH agonist treatment (leuprolide, 3.75 mg subcutaneous injection, per 1 month) to prevent recurrence. On each visit, she was asked if she have hemoptysis and had check-ups including pelvis ultrasonography, chest radiography and CA 125. To date, three cycles of GnRH agonist injection were administered and there was neither recurrent hemoptysis nor the development of new lesions.
Endometriosis is defined as the presence of endometrial glands and stroma beyond the uterine cavity. TES is a type of extra pelvic endometriosis attributable to ectopic (pleural or parenchymal) endometrial tissues. It usually manifests as cyclic pulmonary symptoms such as pneumothorax, hemothorax, hemoptysis, or lung nodules associated with menstruation. Since the first case was reported in 1956 by Lattes et al. [1], 74 cases of CH have been reported in English literature.
The following theoretical mechanisms have been suggested as the pathogenesis of TES: 1) the reflux of endometrial tissue through the fallopian tube and migration via the diaphragmatic defect [23], and 2) cellular embolism of the decidua tissue through the pelvic vessels [45]. The right dominance of the pneumothorax and hemothorax supports the first theoretical mechanism. In an analysis of 110 patients [6], grossly detectable diaphragmatic defects existed in 26% of cases with a pneumothorax and 71% of those with a hemothorax. Meanwhile, the second theoretical mechanism can explain the pulmonary parenchymal involvement of endometrial implants, and it is also supported by endometriosis of the skin or muscle tissue.
In a review of 110 published case reports, Channabasavaiah and Joseph [7] proposed another etiology for TES. They did not find any association with diaphragmatic defects (defined as an artifact rather than a cause of the disease) among 80 cases that had either a pneumothorax or hemothorax. Furthermore, they could not determine a significant relationship between pelvic endometriosis and thoracic endometriosis. These findings suggested that TES is caused by the independent differentiation of non-endometrial stem cells into the endometrial tissue [7].
Generally, TES presents as catamenial pneumothorax, hemothorax, hemoptysis, and asymptomatic lung nodules. The most frequent presentation is a pneumothorax, and CH accounts for 14% of cases. The mean age of patients with CH is 25.9 years, which is significantly lower than that of pneumothorax and hemothorax [7]. There is no clear evidence of the relationship between pelvic and pulmonary endometriosis, but previous cases indicate that pulmonary endometriosis is not always accompanied by pelvic endometriosis.
The diagnosis of CH is usually based on the clinical features. As it is difficult to associate hemoptysis with menses, especially in cases without any evidence of pelvic endometriosis, the diagnosis can be delayed for several months. However, catamenial patterns usually develop within 72 hours after the onset of menses and recurrent hemoptysis. By recognizing such manifestations and excluding other pulmonary causes of hemoptysis, CH can be clinically diagnosed. The serum level of CA 125 may be increased in advanced pelvic endometriosis but in TES, this level is only increased in those with concurrent pelvic endometriosis [8]. Chest radiography is seldom helpful even during the period of hemoptysis, and a bronchoscopy cannot elucidate a specific lesion in most cases. A chest CT scan obtained during menstruation can often identify a ground-glass opacity, consolidation, or nodules, as in our patient [9]. However, the size of the lesion can decrease or even disappear between menses; thus, a comparison serial CT scan may be beneficial.
Thoracic endometriosis can be managed by medicine or surgery. There is no consensus on treatment for preventing symptom recurrence. Historically, surgery was the choice of treatment [10]. Lung wedge resection can be beneficial for recurrent hemoptysis. Sometimes physicians are hesitant to perform surgery due to its invasiveness and associated morbidity. However, with the development of minimally invasive techniques, VATS can reportedly reduce these concerns about surgery. In Korea, since the first reported case of thoracic endometriosis in 2004 [11], several cases have achieved a successful outcome with VATS.
Many authors insist that medicinal treatment should be considered as first-line therapy in patients with non-massive hemoptysis. Current medicinal treatment is designed to suppress ectopic endometrial tissue by blocking estrogen support from the ovary. Commonly used drugs include danazol, oral contraceptives, other progestin agents, and the GnRH agonist. However, with medical treatment alone, recurrence of the disease is very common in even up to 50% of patients [6]. Thus, the effectiveness of combination therapy with operation and adjuvant medication has been reported [1213]. In a single-center analysis of 15 patients, the authors emphasized the importance of adjuvant therapy by using the GnRH agonist following thoracoscopic surgery. Considering the pathogenesis of thoracic endometriosis, eradication of the endometrial plaques followed by suppression of estrogen stimulation is reasonable. The incidence of subcutaneous endometriosis (abdominal wall endometriosis) after cesarean section is estimated to be between 0.03% and 1%. The treatment of choice is wide excision of the lesion with negative margins [14].
Interestingly, in our case, two different types of extra pelvic endometriosis existed. Of course, there is a possibility of undiagnosed pelvic endometriosis because we didn't perform a diagnostic laparoscopy. But even if it exists, such asymptomatic endometriosis can be improved by medical treatment. The patient was young and far from menopause, so she had more chances of recurrence with monthly exposure to estrogen. Therefore, we used a more aggressive treatment with combination therapy. We administered GnRH agonist therapy to prevent additional growth of the ectopic endometrial tissue while she waited for surgery. Then we achieved complete resection of the lesion, and she had a quick recovery since the VATS procedure was used. Leuprolide, a synthetic GnRH analogue, creates reversible hypogonadotropic hypogonadism by causing GnRH receptor down-regulation and is usually used for estrogen-dependent conditions (e.g., endometriosis or uterine fibroids). It is conveniently used as a depot injection monthly, and the side effects are reversible after the medication is discontinued. This medication is safe to use, as we have observed no significant side effects to date.
To our knowledge, this case is the first to report two different types of extra pelvic endometriosis in Korea. TES is an uncommon disease found in reproductive aged women. Although there is no associated mortality reported with TES, it can lower one's quality of life due to symptom recurrence. Even though the long-term follow-up is needed for recurrence, the findings of our case suggest that aggressive combination therapy should be considered in patients who have symptomatic extra pelvic endometriosis.
Figures and Tables
References
1. Lattes R, Shepard F, Tovell H, Wylie R. A clinical and pathologic study of endometriosis of the lung. Surg Gynecol Obstet. 1956; 103:552–558.
2. Slasky BS, Siewers RD, Lecky JW, Zajko A, Burkholder JA. Catamenial pneumothorax: the roles of diaphragmatic defects and endometriosis. AJR Am J Roentgenol. 1982; 138:639–643.
3. Downey DB, Towers MJ, Poon PY, Thomas P. Pneumoperitoneum with catamenial pneumothorax. AJR Am J Roentgenol. 1990; 155:29–30.
4. Inoue T, Kurokawa Y, Kaiwa Y, Abo M, Takayama T, Ansai M, et al. Video-assisted thoracoscopic surgery for catamenial hemoptysis. Chest. 2001; 120:655–658.
5. Park WW. The occurrence of decidual tissue within the lung; report of a case. J Pathol Bacteriol. 1954; 67:563–570.
6. Joseph J, Sahn SA. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996; 100:164–170.
7. Channabasavaiah AD, Joseph JV. Thoracic endometriosis: revisiting the association between clinical presentation and thoracic pathology based on thoracoscopic findings in 110 patients. Medicine (Baltimore). 2010; 89:183–188.
8. Hwang SM, Lee CW, Lee BS, Park JH. Clinical features of thoracic endometriosis: a single center analysis. Obstet Gynecol Sci. 2015; 58:223–231.
9. Kim CJ, Nam HS, Lee CY, Yum HK, Yang SH, Seo KH, et al. Catamenial hemoptysis: a nationwide analysis in Korea. Respiration. 2010; 79:296–301.
10. Rossi NP, Goplerud CP. Recurrent catamenial pneumothorax. Arch Surg. 1974; 109:173–176.
11. Ham HS, Chung MP, Lee BW, Han KH, Kim H, Han J, et al. A case of pulmonary endometriosis resected by video-assisted thoracoscopic surgery. Tuberc Respir Dis. 2004; 56:542–549.
12. Alifano M, Roth T, Broet SC, Schussler O, Magdeleinat P, Regnard JF. Catamenial pneumothorax: a prospective study. Chest. 2003; 124:1004–1008.
13. Azizad-Pinto P, Clarke D. Thoracic endometriosis syndrome: case report and review of the literature. Perm J. 2014; 18:61–65.
14. Horton JD, Dezee KJ, Ahnfeldt EP, Wagner M. Abdominal wall endometriosis: a surgeon's perspective and review of 445 cases. Am J Surg. 2008; 196:207–212.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download