Abstract
Venous thromboembolism is well known as one of the rare but serious adverse effects of combined oral contraceptives (COCs). The COCs with third and fourth generation progestogens were found to have higher risk of venous thrombosis than those with second generation progestogens. We present a case of pulmonary embolism in a 23-year-old nulligravid woman who was using COCs containing the third generation progestogen (desogestrel). At the time of presentation of the adverse effect, she had been using the COCs for 4 months. She had no additional risk factors for thrombosis such as smoking, surgery, tumor as well as genetic factors. This case demonstrates even young women in otherwise good health may be at risk of venous thromboembolism from low-dose formulations of COCs as an over-the-counter drug. We describe this case with a brief review of literatures.
Since Jordan reported a case of venous thrombosis associated with contraceptive use for the first time in 1961 [1], many studies have identified the increased risk of venous thromboembolism (VTE) in current combined oral contraceptive (COC) users [23]. The increase of VTE in current COC users is resulted from the changes in hemostasis. The estrogen component was known to increase plasma levels of coagulation factors and their gene expression, and decrease anticoagulant factors and a tissue factor pathway inhibitor [45]. COCs have been modified in their composition to reduce side effects such as VTE. Apart from lowering the dose of ethinyl estradiol, which is thought to cause the increased risk of venous thrombosis, the progestogen compound has been also changed. Users of COCs with the third and fourth generation progestogens (desogestrel, gestodene, or drospirenone) are generally considered to have higher risk of VTE compared with users of COCs with second generation progestogens (levonorgestrel) [23].
Despite the incidence of VTE including deep vein thrombosis and pulmonary embolism among women of reproductive age is low (about 3 per 10,000 woman-years) [6], the effect of COCs on VTE is significant because of the large number of COC users. Furthermore, the effect of COCs on VTE is important issue considering VTE such as pulmonary embolism may cause a life-threatening condition. Although it has been perceived that VTE is less common in Asian compared with Western people, associated thromboembolism may be thought to increase with recent increase in use of COCs for various purposes other than contraception. Furthermore, COCs with the third generation progestogens are popular as an over-the-counter drug in Korea. We recently encountered a case of pulmonary embolism in a 23-year-old nulligravid Korean woman who was using COCs containing the third generation progestogen (desogestrel), in otherwise no additional risk factors for thrombosis or genetic predisposition. In this article, we report the case with a brief review of concerned literature.
A 23-year-old woman (gravida 0) presented to our emergency room for a chest pain on breathing since 2 days ago. The chest pain began abruptly after working and had been worsening since onset. She complained of a stab of pain from right side of neck to waist on breathing and the pain was relieved in a sitting posture, but aggravated in supine position. Her height was 170 cm and weight was 59 kg. She had a regular menstrual cycle of 28 days, menstruation lasting 5 days. Her menstrual amount was moderate and menstrual pain was tolerable. Her medical and surgical history was unremarkable except that she was taking Mercilon(Merck & Co., Inc., Kenilworth, NJ, USA), a COC containing ethinyl estradiol 0.02 mg and desogestrel 0.15 mg, for contraception. Nine months ago, She had taken Mercilon for three months and then stopped it three months. She started taking Mercilon again during the recent three months. During the recent 3 month she took Mercilon again, she did not have any symptoms at first, but she experienced chest pain and dyspnea at the 3rd months of restart of Mercilon. Her family members had no specific medical history such as a VTE. She was a non-smoker and working as a public servant at office.
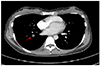
Her initial blood pressure was 154/98 mm Hg, pulse rate 128/min, respiratory rate 20/min, body temperature 36.5℃. Auscultation revealed a coarse breathing sound and rale in right lower lung, but other abnormalities were not detected on physical examination. There were no skin color changes, edema or asymmetry of both legs. Laboratory finding showed D-dimer was elevated as 1.65 µg/mL (normal, up to 0.55 µg/mL) and protein S activity was decreased as 42.3% (normal, 60% to 130%). Other tests including Lupus anticoagulant, antiphospholipid antibody IgM, and anti-cardiolipin antibody IgM were all normal. Chest computed tomography (CT) showed acute pulmonary thromboembolism involving basal segmental branch of right lower lobe and subpleural consolidation and ground glass opacity in the posterobasal segment of right lower lobe suggesting pulmonary infarction and hemorrhage (Fig. 1). Lower extremities vein doppler revealed no evidence of deep vein thrombosis in both lower extremities. She got low molecular weight heparin injections (10,000 IU/day) subcutaneously for 5 days and took 20 mg rivaroxaban daily per oral for 6 months thereafter.
She had regular menstrual cycle with no complaints and no recurred pulmonary thromboembolism at the time of follow-up after 18 months.
VTE is a multifactorial disease that represents the interaction of genetic and acquired factors. In addition to COCs, other acquired risk factors such as obesity, smoking, surgery, trauma, and acute or chronic medical illnesses are more common than inherited thrombophilia in the development of VTE, but heredity is also an important predisposing factor. Therefore, VTE is a common condition with significant morbidity and mortality in Western countries where inherited thrombophilia are more common than Asian. Studies from various communities have reported the incidence of VTE is 100 cases or greater per 100,000 person-years in Western countries, whereas 17 cases per 100,000 person-years in Asian people [78].
In this study, we reported a case of pulmonary embolism in a 23-year-old nulligravid woman who was using COC in otherwise no additional risk factors for thrombosis. Clinically, it has been postulated that pulmonary embolism is a sequel of deep vein thrombosis as clots, formed in the lower extremity, break off and move to the pulmonary vasculature [9], but the lower extremities vein doppler ultrasound showed no evidence of deep vein thrombosis in this case. Considering deep vein thrombosis and pulmonary embolism share a common pathogenesis based on Virchow's triad of blood flow stasis, vessel wall damage and increased blood viscosity, and they are part of the same disease, VTE, pulmonary embolism can occur under the conditions that predispose to VTE without the evidence of deep vein thrombosis. Pulmonary embolism may present as sudden death or atypical symptoms such as cough, substernal chest pain, hemoptysis, and wheezing, but a recent survey reported that the most common clinical symptoms were dyspnea (78% to 81%), pleuritic chest pain (39% to 56%), and fainting or syncope (22% to 26%) [10]. Clinical signs such as tachypnea (≥20 breaths/min) and tachycardia (>100 beats/min) that could suspect the pulmonary embolism lack sensitivity and specificity for the confirmative diagnosis of pulmonary embolism [11] and some cases of pulmonary embolism are recognized by CT scans incidentally without symptoms [12]. The 2008 European Society of Cardiology guidelines for the management of pulmonary embolism recommended initial diagnostic stratification based on mortality risk before confirmatory diagnostic tests [13]. In cases of high-risk pulmonary embolism, confirmatory diagnosis of pulmonary embolism is made by CT pulmonary angiography (CTPA) or echocardiography (if CTPA is not available) and immediate anticoagulation with intravenous unfractionated heparin and thrombolytic therapy with surgical embolectomy as a back-up are recommended, whereas in cases of non-high-risk pulmonary embolism, laboratory tests including D-dimer and other investigations such as chest X-ray, levels of arterial blood gases and electrocardiography that can exclude other causes for signs and symptoms like pulmonary embolism are performed at first before CTPA. Anticoagulation therapy traditionally with parenteral heparins in the acute phase of treatment, transitioning to oral vitamin K antagonists is recommended for non-high-risk presentation of pulmonary embolism. To date, non-vitamin K antagonist oral anticoagulants after parenteral anticoagulant induction were reported that they are non-inferior to enoxaparin/vitamin K antagonists for the prevention of recurrent VTE, reducing the risk of major bleeding [14]. In this case, the patient could be categorized as a low-to moderate clinical probability of non-high-risk pulmonary embolism and we could make a diagnosis by blood tests including D-dimer and chest CT scanning. And she was treated low molecular weight heparin for 5 days followed by rivaroxaban, non-vitamin K antagonist oral anticoagulants for 6 months.
It was reported that the effect size of VTE risk among COCs users depended both on the dose of ethinyl estradiol and the progestogen used [3]. It is unclear why the different progestogen provided makes difference in the VTE risk of COCs, but a possibility is that there is a difference in inhibitory effects of progestogen on the procoagulant effect of ethinyl estradiol. As an example, desogestrel users made more pronounced effects on coagulation factors of the COCs when compared with the effects in levonogestrel users [15].
Considering COCs with the third generation progestogens such as desogestrel are popular as an over-the-counter drug in Korea, it should be kept in mind that all COCs can increase the risk of thrombosis. Moreover, the risk of VTE is known to increase during the first 4 months of COCs use and decline thereafter, and is higher even in women restarting COCs use after a break [2]. This case suggests that common wrong practice to have intentional drug holiday in the long-term COCs users in Korea can increase the risk of VTE again. This case also reminds us that even young woman without any risk factors or genetic predisposition for thrombosis may develop pulmonary embolism from low-dose formulations of COCs.
Figures and Tables
References
1. Jordan WM. Pulmonary embolism. Lancet. 1961; 278:1146–1147.
2. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1995; 346:1575–1582.
3. Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013; 347:f5298.
4. Bladbjerg EM, Skouby SO, Andersen LF, Jespersen J. Effects of different progestin regimens in hormone replacement therapy on blood coagulation factor VII and tissue factor pathway inhibitor. Hum Reprod. 2002; 17:3235–3241.
5. Suzuki A, Sanda N, Miyawaki Y, Fujimori Y, Yamada T, Takagi A, et al. Down-regulation of PROS1 gene expression by 17beta-estradiol via estrogen receptor alpha (ERalpha)-Sp1 interaction recruiting receptor-interacting protein 140 and the corepressor-HDAC3 complex. J Biol Chem. 2010; 285:13444–13453.
6. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007; 5:692–699.
7. Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008; 28:370–372.
8. Cohen A, Chiu KM, Park K, Jeyaindran S, Tambunan KL, Ward C, et al. Managing venous thromboembolism in Asia: winds of change in the era of new oral anticoagulants. Thromb Res. 2012; 130:291–301.
9. Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005; 365:1163–1174.
10. Miniati M, Cenci C, Monti S, Poli D. Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS One. 2012; 7:e30891.
11. Cohen AT, Dobromirski M, Gurwith MM. Managing pulmonary embolism from presentation to extended treatment. Thromb Res. 2014; 133:139–148.
12. Douma RA, Kok MG, Verberne LM, Kamphuisen PW, Buller HR. Incidental venous thromboembolism in cancer patients: prevalence and consequence. Thromb Res. 2010; 125:e306–e309.
13. Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008; 29:2276–2315.
14. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:2 Suppl. e419S–e494S.
15. Kemmeren JM, Algra A, Meijers JC, Bouma BN, Grobbee DE. Effects of second and third generation oral contraceptives and their respective progestagens on the coagulation system in the absence or presence of the factor V Leiden mutation. Thromb Haemost. 2002; 87:199–205.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download