Abstract
Ovarian mucinous tumors in <15 years old are rare with <50 cases reported till date in the literature. Majority of them are benign or borderline epithelial ovarian tumors with only 12 cases of cystadenocarcinomas reported at a young age. We report a case of mucinous cystadenocarcinoma in 14-year-old girl with metastasis to omentum at the time of presentation. Management of such cases is tricky as conservative approach sparing fertility of the patient is adopted. This case is presented for its rarity and unique presentation. To the best of our knowledge, this is the thirteenth case of ovarian cystadenocarcinoma being reported at a very young age and the first case being reported from Indian subcontinent. Extensive review of the previously published cases in the literature has been done in this study.
Epithelial ovarian tumors are rare in children comprising 15% to 20% of cases [1]. Not surprisingly benign cystadenomas are common in younger age group and malignant lesions are rare. We present a case of 14-year-old girl with ovarian mucinous cystadenocarcinoma presenting with metastasis. To the best of our knowledge, this is the thirteenth case of ovarian cystadenocarcinoma being reported at such a young age in the world literature and the first case being reported from Indian subcontinent.
A 14-year-old girl presented to gynecology out patient department with complaints of abdominal distension for past 3 months. Distension was insidious in onset and gradually increasing in size with presence of abdominal pain for past 1 week. Past history was unremarkable. Patient had irregular menses with oligomenorrhoea for past 3 months. General physical examination was unremarkable.
Abdominal examination showed generalized abdominal distension from epigastric to hypogastric region with presence of fluid thrill. No hepatosplenomegaly was noted. Vaginal examination revealed uterus in anteverted position with free fornices.
Tumor markers were evaluated and showed marked elevation in CA 125 level of 243 U/L (normal, 0 to 35 U/L). β-human chorionic gonadotropin, alpha feto protein, carcino embryonic antigen and lactate dehydrogenase were within normal limits. Complete hemogram, liver function test and coagulation profile were normal.
Plain X-ray chest and abdomen revealed a solid cystic lesion occupying whole of the abdomen. Contrast enhanced computed tomography scan of abdomen revealed well defined multi-loculated predominantly cystic abdominopelvic intraperitoneal mass lesion with enhancing septas and solid components within and extending from sub-hepatic location to pubic symphysis inferiorly.
Patient underwent explorative laparotomy and a large left sided ovarian tumor measuring 20×17 cm with intact capsule was noted adhered to the omentum however surrounding peritoneum and contralateral ovary appeared unremarkable. Two hundred milliliters of ascitic fluid was aspirated and sent for cytological examination for malignant cells. In addition, macroscopic deposits were seen in the omentum. Left sided salphingo-oophorectomy was done and sent for frozen section. Subsequently patient underwent infracolic omentectomy and the specimen was sent for routine histopathological examination. Frozen section findings revealed an enlarged ovary measuring 20×10×5 cm with intact capsule. Cut section was multi-loculated with presence of solid cystic areas. Cysts ranged in size from 0.5 to 4.5 cm and were filled with thick, viscous mucinous fluid (Fig. 1A). Microscopy revealed ovarian stroma with suspicious atypical cells lying in the pools of mucin, however no definite invasion was seen in the stroma. Therefore, possibility of borderline mucinous tumor was suggested.
Microscopic sections, on routine histological examination, revealed solid cystic areas displaying multiple layers of mucin producing atypical epithelium, with large areas of necrosis, focally infiltrating the stroma (Fig. 1B). Sections from the omentum revealed metastatic tumor deposits. Thus, a final diagnosis of malignant mucinous cystadenocarcinoma with metastasis to omentum was rendered. Ascitic fluid cytology was positive for malignant cells.
Postoperative course of the patient was unremarkable with fall in CA 125 levels. Patient was given 3 cycles of chemotherapy with cisplatin and paclitaxel. Eight months postsurgery, patient showed no signs of recurrence and is still under follow up.
Mucinous ovarian tumors are rare in children <15 years of age with <50 cases reported in world literature [12345678910]. Majority of these are benign/borderline tumors with frequency of carcinoma still rarer with only twelve cases reported at a young age of <15 years [23457910] (Table 1).
Mostly, patients present with vague symptoms that are initially ignored by them. This often leads to advancement of disease before the lesion gets diagnosed. In our case too, patient initially ignored the symptoms and had already developed metastasis at the time of presentation. Furthermore, it is believed that childhood tumors are far more aggressive than their adult counterparts and progress to advanced disease despite treatment [7].
Most of the cases of adenocarcinoma reported in the literature are pre-menarchal though some of them hover around the age group of 14 years. Our patient was however post menarchal. This difference can be explained on the basis of international variability of attaining menarche. Our patient was an Asian origin girl whereas most of the other studies have been reported on American or European girls.
Tumor markers and radiology in collaboration serve as an essential tool in the diagnosis of ovarian cancers [6]. CA 125 has been widely used as a marker for epithelial ovarian tumors however its utility is debatable. Although elevated serum CA 125 levels (>35 U/mL) have been found in more than 80% of ovarian cancer patients, only 50% of patients with stage I disease have elevated levels. Furthermore, CA 125 is also raised in approximately 1% of healthy control subjects, liver cirrhosis, endometriosis, first-trimester pregnancy, pelvic inflammatory disease, pancreatitis, and in 40% of patients with advanced intra-abdominal non-ovarian malignancy [11]. Therefore, its raised levels must always be interpreted with caution and in conjunction with radiology. It is believed that if levels are initially raised at the time of detection they can be used as a marker for identifying residual or recurrent disease later at follow up [68].
Sometimes mass can be very big creating confusion as to the exact source of origin even on radiology. In our case too, huge intra-abdominal mass along with ascites created problems in identifying the exact site of origin and therefore contrast enhanced computed tomography scan was advised. Raised CA 125 levels in collaboration with the computed tomography scan report lead clinicians to believe that they were dealing with an ovarian mass probably epithelial in origin and explorative laparotomy was performed.
The intra-operative examination of the other ovary and surrounding structures is important as it can often lead to upgradation of the stage of the tumor if macroscopic deposits are visualized during surgery. Though the adult staging protocols dictate mandatory lymphnode dissections and biopsies of peritoneal surfaces; these procedures are often omitted in pediatric cases unless gross metastatic disease is present [10]. In our patient, the other ovary, uterus, fallopian tube and pouch of Douglas were unremarkable. However, macroscopic deposits were seen in the omentum and histopathological examination of infracolic omentectomy specimen confirmed the suspicion and upgraded the tumor to stage III by FIGO (International Federation of Gynecology and Obstetrics) guidelines. Given the significant incidence of bilateral disease, some authors have also recommended a prophylactic wedge biopsy of the uninvolved ovary at the time of initial debulking surgery or biopsy from grossly suspicious foci; however this was not performed in the present case.
Intraoperative frozen section can often aid in the diagnosis and further management of the patient. However, it can often be challenging to report a carcinoma in a minor age group without definitive infiltration into the stroma, rendering an equivocal report, as in our case.
Guidelines of surgical treatment of malignant ovarian tumor with metastasis is an aggressive surgery in adults comprising of total abdominal hysterectomy with bilateral salphingo-oopherectomy along with tumor debulking/cytoreduction. Rationale of doing a fertility sparing surgery inspite of possible omental deposits in present case lies in the fact that fertility is a very important issue in young patients. Furthermore, some studies have compared fertility sparing surgery with radical surgery in borderline ovarian tumors [12]. In these studies, though the recurrence rate was some-what higher in the fertility sparing group as compared to radical group, these recurrences were amenable to salvage by subsequent surgeries.
A study done by Aggarwal et al. [13] pointed out that low malignant recurrences have been reported more than ten years after initial surgery even in an adult patient population. Therefore, like in adults; young patients should also be kept under close follow up to monitor recurrence which should be treated with another salvage surgery.
Various adjuvant regimens ranging from single agent (carboplatin) [14] to multi agent regime have been tried for the treatment of malignant ovarian neoplasms. More recently, combination of hexamethylmelamine, doxorubicin, and cis-diamminedicholoroplatinum with/without methotrexate has been used successfully in adults for the treatment [215]. Whereas, Blom and Torkildsen [3] administered intraperitoneal phosphorus in the paediatric mucinous cystadenocarcinoma that they encountered; Gribbon et al. [4] gave intraperitoneal radiotherapy in their two cases of cystadenocarcinomas. In the present case, the patient received 3 cycles of chemotherapy with cisplatin and paclitaxel.
Prognosis of ovarian cancers presenting at younger age remains variable and depends on the stage of presentation. Most of the cases reported in the literature have had bad prognosis with almost all the patients dying within five years of detection of the lesion. Prognosis of our case too seemed dismal since it had already metastasized at the time of diagnosis.
Epithelial ovarian tumors rarely occur in children <15 years of age, and are always almost benign. Malignant neoplasms are exceedingly rare however should always be kept in mind especially in cases with raised CA 125 levels suggesting non-germ cell origin. Since signs and symptoms are vague, patient often presents late and at an advanced stage. Management of these cases differ from their adult counterparts as instead of radical surgery, fertility sparing conservative approach should be adopted in these cases.
References
1. Horiuchi A, Kameoka K, Sato K, Yamamoto Y, Watanabe Y. Huge mucinous borderline ovarian cystadenoma in a premenarchal girl. Open J Pediatrics. 2012; 2:82–86.

2. Hong SJ, Lurain JR, Tsukada Y, Piver MS, Humbert JR, Freeman AI. Cystadenocarcinoma of the ovary in a 4-year-old: benign transformation during therapy. Cancer. 1980; 45:2227–2230. PMID: 7370965.

3. Blom GP, Torkildsen EM. Ovarian cystadenocarcinoma in a 4-year-old girl: report of a case and review of the literature. Gynecol Oncol. 1982; 13:242–246. PMID: 7076039.

4. Gribbon M, Ein SH, Mancer K. Pediatric malignant ovarian tumors: a 43-year review. J Pediatr Surg. 1992; 27:480–484. PMID: 1326038.

5. Skinner MA, Schlatter MG, Heifetz SA, Grosfeld JL. Ovarian neoplasms in children. Arch Surg. 1993; 128:849–853. PMID: 8343057.

6. Deprest J, Moerman P, Corneillie P, Ide P. Ovarian borderline mucinous tumor in a premenarchal girl: review on ovarian epithelial cancer in young girls. Gynecol Oncol. 1992; 45:219–224. PMID: 1592292.

7. Shankar KR, Wakhlu A, Kokai GK, McDowell H, Jones MO. Ovarian adenocarcinoma in premenarchal girls. J Pediatr Surg. 2001; 36:511–515. PMID: 11227010.

8. Iwasaki M, Taira K, Kobayashi H, Saiga T. Ovarian mucinous cystadenoma of borderline malignancy in a premenarchal girl. J Pediatr Adolesc Gynecol. 2010; 23:e119–e123. PMID: 19896401.

9. Hernandez E, Rosenshein NB, Parmley TH. Mucinous cystadenocarcinoma in a premenarchal girl. South Med J. 1982; 75:1265–1267. PMID: 7123301.

10. Morowitz M, Huff D, von Allmen D. Epithelial ovarian tumors in children: a retrospective analysis. J Pediatr Surg. 2003; 38:331–335. PMID: 12632344.

11. Zanaboni F, Vergadoro F, Presti M, Gallotti P, Lombardi F, Bolis G. Tumor antigen CA 125 as a marker of ovarian epithelial carcinoma. Gynecol Oncol. 1987; 28:61–67. PMID: 2443433.

12. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Surgical management of borderline ovarian tumors: the role of fertility-sparing surgery. Gynecol Oncol. 2009; 113:75–82. PMID: 19171373.

13. Aggarwal A, Lucco KL, Lacy J, Kives S, Gerstle JT, Allen L. Ovarian epithelial tumors of low malignant potential: a case series of 5 adolescent patients. J Pediatr Surg. 2009; 44:2023–2027. PMID: 19853767.

14. ICON Collaborators. International Collaborative Ovarian Neoplasm Study. ICON2: randomised trial of single-agent carboplatin against three-drug combination of CAP (cyclophosphamide, doxorubicin, and cisplatin) in women with ovarian cancer. Lancet. 1998; 352:1571–1576. PMID: 9843101.
15. Brower MS, Coleman M, Pasmantier MW, Silver RT, Mamaril AP, Quiguyan CC. Treatment of advanced ovarian carcinoma with hexamethylmelamine, doxorubicin, and cis-platinum (HAC): results in both untreated and previously treated patients. Med Pediatr Oncol. 1984; 12:17–24. PMID: 6321930.
Fig. 1
(A) Gross: ovarian cyst. Cut section is solid cystic with multi-loculated cysts filled with mucinous material. (B) Microscopy: back to back arrangement of glands lined by mucin secreting epithelium infiltrating the stroma (H&E, ×200).
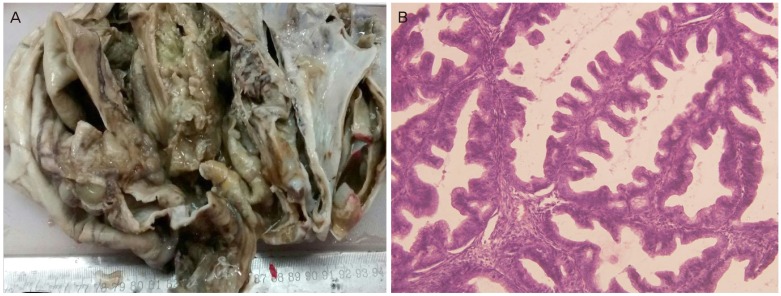
Table 1
Ovarian cystadenocarcinoma reported in the literature in <15 years of age
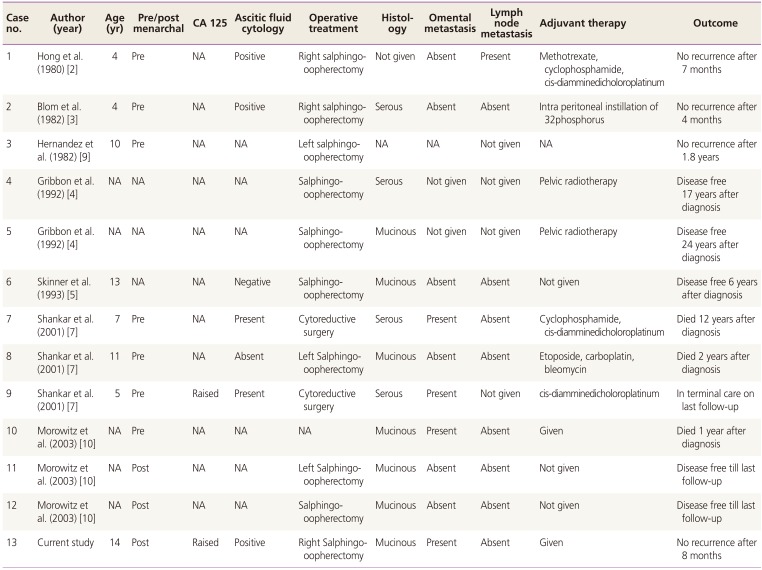
| Case no. | Author (year) | Age (yr) | Pre/post menarchal | CA 125 | Ascitic fluid cytology | Operative treatment | Histology | Omental metastasis | Lymph node metastasis | Adjuvant therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hong et al. (1980) [2] | 4 | Pre | NA | Positive | Right salphingo-oopherectomy | Not given | Absent | Absent | Methotrexate, cyclophosphamide, cis-diamminedicholoroplatinum | No recurrence after 7 months |
| 2 | Blom et al. (1982) [3] | 4 | Pre | NA | Positive | Right salphingo-oopherectomy | Serous | Absent | Absent | Intra peritoneal instillation of 32phosphorus | No recurrence after 4 months |
| 3 | Hernandez et al. (1982) [9] | 10 | Pre | NA | NA | Left salphingo-oopherectomy | NA | NA | Not given | NA | No recurrence after 1.8 years |
| 4 | Gribbon et al. (1992) [4] | NA | NA | NA | NA | Salphingo-oopherectomy | Serous | Not given | Not given | Pelvic radiotherapy | Disease free 17 years after diagnosis |
| 5 | Gribbon et al. (1992) [4] | NA | NA | NA | NA | Salphingo-oopherectomy | Mucinous | Not given | Not given | Pelvic radiotherapy | Disease free 24 years after |
| 6 | Skinner et al. (1993) [5] | 13 | NA | NA | Negative | Salphingo-oopherectomy | Mucinous | Absent | Absent | Not given | Disease free 6 years after diagnosis |
| 7 | Shankar et al. (2001) [7] | 7 | Pre | NA | Present | Cytoreductive surgery | Serous | Present | Absent | Cyclophosphamide, cis-diamminedicholoroplatinum | Died 12 years after diagnosis |
| 8 | Shankar et al. (2001) [7] | 11 | Pre | NA | Absent | Left Salphingo-oopherectomy | Mucinous | Absent | Absent | Etoposide, carboplatin, bleomycin | Died 2 years after diagnosis |
| 9 | Shankar et al. (2001) [7] | 5 | Pre | Raised | Present | Cytoreductive surgery | Serous | Present | Not given | In terminal care on last follow-up | In terminal care on last follow-up |
| 10 | Morowitz et al. (2003) [10] | NA | Pre | NA | NA | NA | Mucinous | Present | Absent | Given | Died 1 year after diagnosis |
| 11 | Morowitz et al. (2003) [10] | NA | Post | NA | NA | Left Salphingo-oopherectomy | Mucinous | Absent | Absent | Not given | Disease free till last follow-up |
| 12 | Morowitz et al. (2003) [10] | NA | Post | NA | NA | Salphingo-oopherectomy | Mucinous | Absent | Absent | Not given | Disease free till last follow-up |
| 13 | Current study | 14 | Post | Raised | Positive | Right Salphingo-oopherectomy | Mucinous | Present | Absent | Given | No recurrence after 8 months |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download