This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
This study aimed to introduce a method to remove huge ovarian tumors (≥15 cm) intact with single-port laparoscopic surgery (SPLS) using SW Kim's technique and to compare the surgical outcomes with those of laparotomy.
Methods
Medical records were retrospectively reviewed for patients who underwent either SPLS (n=21) with SW Kim's technique using a specially designed 30×30-cm2-sized 3XL LapBag or laparotomy (n=22) for a huge ovarian tumor from December 2008 to May 2016. Perioperative surgical outcomes were compared.
Results
In 19/21 (90.5%) patients, SPLS was successfully performed without any tumor spillage or conversion to multi-port laparoscopy or laparotomy. There was no significant difference in patient characteristics, including tumor diameter and total operation time, between both groups. The postoperative hospital stay was significantly shorter for the SPLS group than for the laparotomy group (median, 2 [1 to 5] vs. 4 [3 to 17] days; P<0.001). The number of postoperative general diet build-up days was also significantly shorter for the SPLS group (median, 1 [1 to 4] vs. 3 [2 to 16] days; P<0.001). Immediate post-operative pain score was lower in the SPLS group (median, 2.0 [0 to 8] vs. 4.0 [0 to 8]; P=0.045). Patient-controlled anesthesia was used less in the SPLS group (61.9% vs. 100%).
Conclusion
SPLS was successful in removing most large ovarian tumors without rupture and showed quicker recovery and less immediate post-operative pain in comparison to laparotomy. SPLS using SW Kim's technique could be a feasible solution to removing huge ovarian tumors.
Go to :

Keywords: Laparoscopy, Ovarian cysts, Single port
Introduction
Large ovarian tumors are frequently encountered in the gynecological field. Currently, laparotomy is regarded as a standard procedure for large ovarian tumor surgery. Although laparoscopy has advantages over laparotomy, including a shorter length of hospital stay, a faster return to normal activities, aesthetic enhancement, and reduced postoperative pain [
12], laparoscopic surgery for a huge ovarian tumors is still in under debate due to potential malignancy and technical difficulties [
3]. Recently, with the demand of more minimally invasive surgery, single-port laparoscopic surgery (SPLS) has been introduced in gynecology and has improved with advances in surgical instruments and techniques. With the scar hidden in the umbilicus, SPLS has been performed in patients with an ovarian cyst [
456789]. This technique has generated considerable interest for ovarian cyst surgery, as it has favorable outcomes and stable results [
48]. The benefits of SPLS are reduced infection rate, less blood loss, shorter recovery time, and cosmetic satisfaction for reduced incision size compared to multiport laparoscopy or laparotomy [
10]. There have been several recent reports describing limited case numbers [
56] and relatively small tumor size (largest diameter 12 cm) [
47910]. Song et al. [
8] reported outcomes of SPLS in extremely large ovarian cysts; however, rather than removing the tumor intact, they aspirated the cystic fluid inside the abdominal cavity and then removed the cyst through the wound retractor without using a tumor retrieval bag. Therefore, their method could not be applied to large ovarian tumors, as it could potentially spread hidden malignancy. There is still no report that describes a method for removing ovarian tumors 20 to 30 cm in diameter without aspiration or rupture, not only in conventional laparoscopy but also in SPLS.
In this study, we aimed to introduce SW Kim's technique for SPLS to remove a huge ovarian tumor without tumor spillage or cystic fluid aspiration. The surgical outcomes of SPLS using SW Kim's technique were compared to those of laparotomy.
Go to :

Materials and methods
1. Patient details
We retrospectively reviewed medical records of patients who underwent SPLS using SW Kim's technique or laparotomy for an ovarian tumor between December 2008 and May 2016 in the Department of Obstetrics and Gynecology at Severance Hospital, Yonsei University College of Medicine in Seoul, Korea. We selected patients with a large ovarian tumor (longest diameter of ≥15 cm) without evidence of definitive malignancy on preoperative evaluation. The largest length of the ovarian cyst was measured with computerized tomography or magnetic resonance imaging scans. Patients who underwent concurrent surgery in other organs were excluded, as were those with existing symptomatic gynecological conditions that may influence pain intensity, such as tubo-ovarian abscess, ovarian cyst torsion, ruptured tubal pregnancy, or hemorrhagic cysts with hemoperitoneum. We enrolled 72 patients with ovarian cysts with a diameter ≥10 cm. We then selected 45 patients with ovarian cysts with a diameter ≥15 cm. Two patients were then excluded due to misdiagnosis (retroperitoneal mass, not ovarian cyst) and absence of medical records. In total, 43 patients (21 patients in the SPLS group and 22 patients in the laparotomy group) were included in this study.
Patient characteristics, including age, body mass index, previous abdominal surgeries, and surgical outcomes, including operation time, estimated blood loss, postoperative hemoglobin change, days of postoperative hospital stay, postoperative general diet build-up day, and postoperative pain score data were collected. Operation time was defined as the time from umbilical incision to completion of skin closure. Estimated blood loss was calculated by the anesthesiology unit as the difference between the total amount of suction and irrigation plus the difference between the total gauze weight before and after surgery. Hemoglobin change was defined as the difference between preoperative hemoglobin and hemoglobin at postoperative day 1. Days of postoperative hospital stay was defined as the number of days from operation to discharge. General diet build-up day was defined as the number of days from surgery until the patient is allowed to have a general diet. Pain score was measured using the visual analogue scale. The visual analogue scale is a psychometric response scale that can be used in questionnaires and describes postoperative pain intensity as no (0 to 4 mm), mild (5 to 44 mm), moderate (45 to 74 mm), and severe (75 to 100 mm) pain. We asked postoperative patients to depict the score at the recovery room (immediate postoperative) and 6, 24, and 48 hours later. This study was approved by institutional review board at Yonsei University College of Medicine.
2. Single port laparoscopy
As reported in our previous study [
11], the Alexis wound retractor (Applied Medical, Rancho Santa Margarita, CA, USA) and surgical glove were used for the SPLS entry system, with a specially designed 30×30-cm
2-sized laparoscopic specimen retrieval bag (3XL LapBag, Sejong Medical Co., Seoul, Korea). We used a 45-cm-long, rigid 30°, 5 mm endoscope for SPLS. The key surgical instruments for SPLS were a monopolar L-hook, biopsy forceps with a slightly bent shaft, two needle holders, and LigaSure (Covidien Valleylab, Boulder, CO, USA) (
Fig. 1). Upon entering the abdominal cavity, peritoneal washings were obtained for cytology. We then performed an ovarian cystectomy or unilateral salpingo-oophorectomy without rupture or aspiration of cystic fluid, safely removing the large ovarian tumor using SW Kim's technique. Large ovarian tumors usually occupy the pelvic cavity, making it difficult to identify the pelvic anatomy without changing the patient's position (
Figs. 2A,
5A). However, large ovarian tumors can be moved away from the pelvic cavity to the upper abdominal cavity by adjusting the table to a Trendelenburg position. The tumor falls into the upper abdominal cavity due to gravity and the infundibulopelvic ligament can be exposed (
Fig. 2B). For salpingo-oophorectomies, the infundibulopelvic ligament is ligated and dissected with LigaSure (
Fig. 2C). After ligating the utero-ovarian ligament, the patient's position is changed to the reverse Trendelenburg position so that the tumor falls into the pelvic cavity (
Fig. 2D). Using SW Kim's technique, the tumor can be safely inserted into the 3XL LapBag.
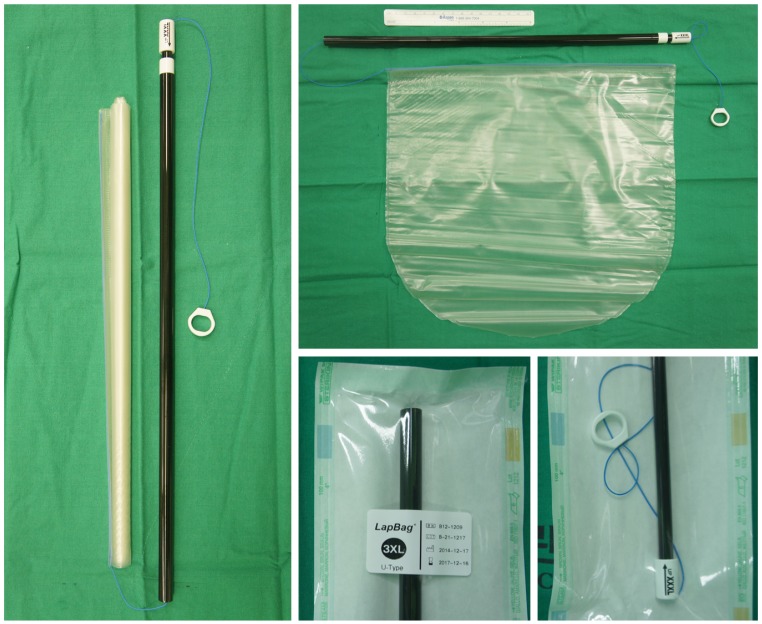 | Fig. 1A specifically designed 30×30-cm2-sized laparoscopic specimen retrieval bag (3XL LapBag, Sejong Medical Co., Seoul, Korea).
|
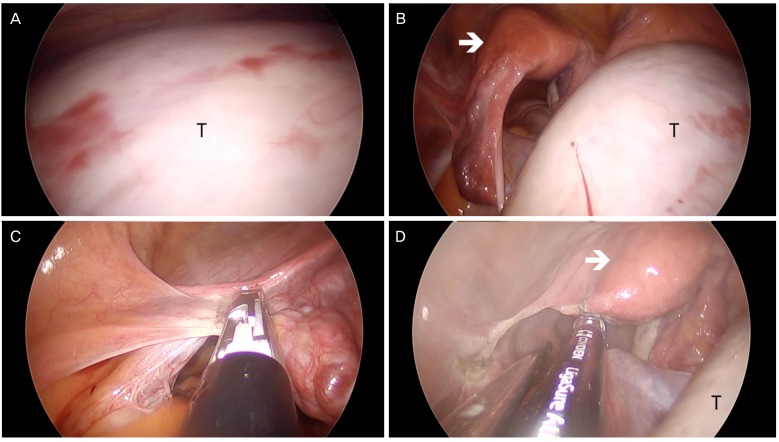 | Fig. 2Intraoperative view of single-port laparoscopic surgery in a patient with a large left ovarian cyst tumor. (A) A 22-cm left ovarian cystic tumor (T) is occupying the entire lower abdominal cavity in supine position. (B) In the Trendelenburg position, the tumor goes into the upper abdominal cavity, and the uterus (arrow) and left infundibulopelvic ligament are exposed. (C) The left infundibulopelvic ligament is ligated and dissected with a LigaSure. (D) The left utero-ovarian ligament and fallopian tube are ligated with a LigaSure.
|
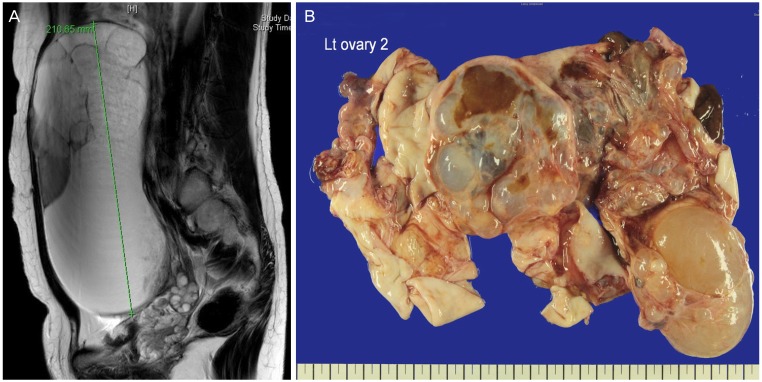 | Fig. 5(A) A large ovarian cystic tumor is occupying the lower abdominal cavity (T2 magnetic resonance image). (B) Gross finding of a large ovarian cystic tumor after retrieval using a 3XL LapBag. Lt, left.
|
3. SW Kim's technique
SW Kim's technique is composed of 5 parts: 1) 3XL LapBag insertion, 2) 3XL LapBag unfolding, 3) grasping of the bag opening in a triangular shape using two needle holders and one grasper and approaching the bag opening to the tumor, 4) insertion of the tumor into a 3XL LapBag by changing patient's position to the Trendelenburg position, and 5) tumor removal through the umbilicus inside the 3XL LapBag.
Fig. 3A shows the 3XL LapBag inserted into the pelvic cavity over the tumor. After unfolding the 3XL LapBag above the tumor (
Fig. 4A), the bag is moved to the upper abdominal cavity. The bag opening is then made into a triangular shape by holding three apexes using graspers. For holding the bilateral bottom corner of the bag, needle holders are used to fix the bag while placing the tumor into the bag (
Figs. 3B,
4B). The lower edge of the bag is positioned under the ovarian tumor and the center of the upper edge of the bag is held with a grasper so that the bag opening is kept in a triangular shape (
Fig. 3C). The triangular shaped bag opening is then placed to the cranial portion of the tumor and the patient is put in the Trendelenburg position. The bag is then pulled over the tumor and the tumor falls into the bag (
Figs. 3D,
4C, and 4D). The tumor is then removed through the umbilical incision or vagina inside the bag (
Fig. 5B).
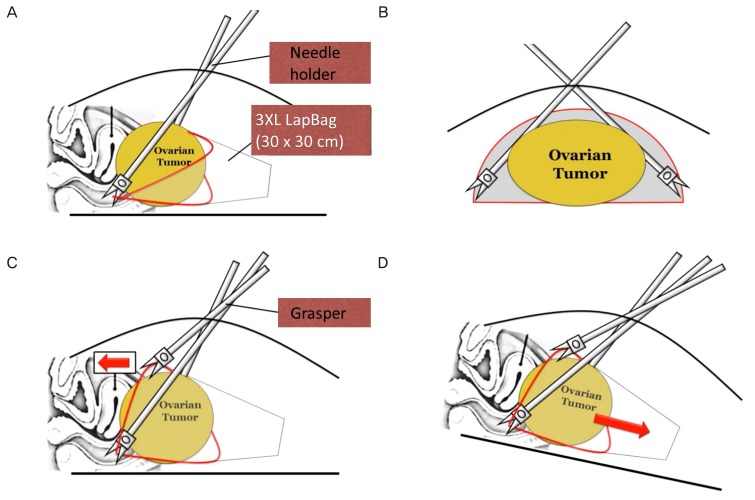 | Fig. 3SW Kim's technique for placing a large tumor in a laparoscopic bag (3XL LapBag). After completing salpingo-oophorectomy, the ovarian tumor is located into the pelvic cavity by changing the patient's position into reverse Trendelenburg position. Then insert the 3XL LapBag into the pelvic cavity. After unfolding the 3XL LapBag above the tumor, the bag is taken into the upper abdominal cavity. The bag opening is made into a triangular shape by holding three apexes by graspers. (A) For holding the bilateral bottom corner of the bag, needle holders are used to grasp the bag because it can firmly hold the bag without loosening. The lower edge is positioned under the ovarian tumor. (B) Transverse view: the lower edge is held with two needle holders and positioned under the ovarian tumor. (C) The center of the upper edge of the bag is held with a grasper and the opening is made into a triangular shape. The bag opening is placed on the cranial portion of the tumor and pulled over the ovarian tumor while the patient is changed into Trendelenburg position. (D) In Trendelenburg position, the bag is pulled over the tumor, which falls into the bag because of gravity.
|
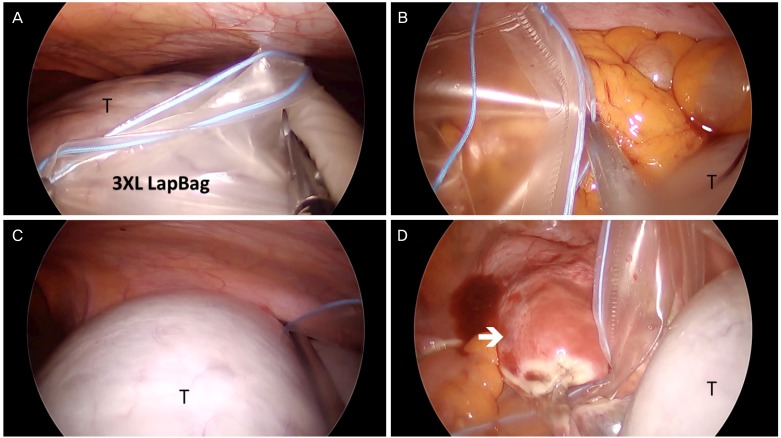 | Fig. 4Intraoperative view of SW Kim's technique for placing a large tumor in a laparoscopic bag (3XL LapBag). (A) A 3XL LapBag is inserted into the lower abdominal cavity and unfolded over the tumor (T). (B) The left bottom corner of the bag is held with a needle holder. (C) The right bottom corner of the bag is held with a needle holder and the tumor is goes into the bag by moving the patient into the Trendelenburg position. (D) The tumor is placed in the bag (arrow: uterus).
|
4. Statistics
For statistical analysis, SPSS ver. 18 (SPSS Inc., Chicago, IL, USA) was used. The Mann-Whitney U-test and Pearson's chi-squared test were used to analyze the nonparametric variables. A median with a range was used to describe the distribution of data. All P-values <0.05 were considered statistically significant and all reported P-values were two-sided.
Go to :

Results
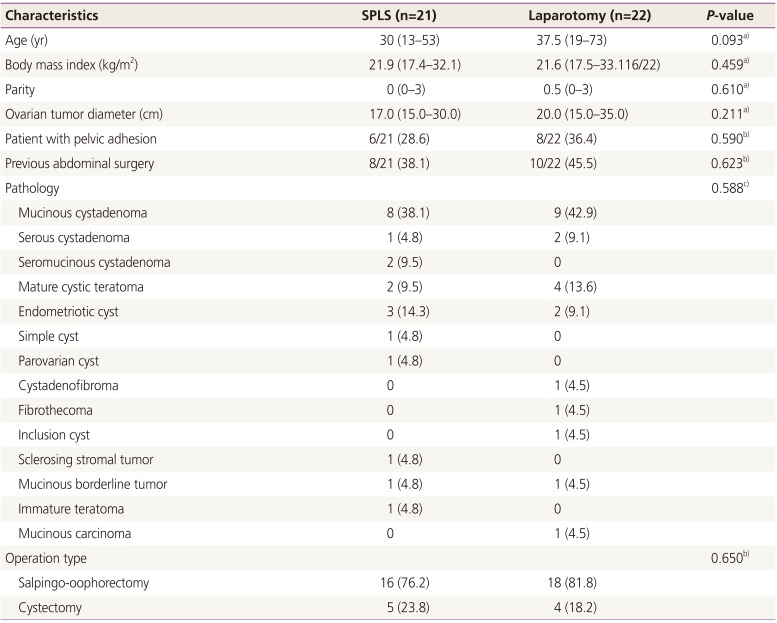
In this study, 43 patients who underwent SPLS using SW Kim's technique or laparotomy for an extremely huge ovarian tumor (diameter of ≥15 cm) were selected. Patient characteristics are summarized in
Table 1. The most common pathology was mucinous cystadenoma (38.1% in SPLS and 42.9% in laparotomy). There were two malignant cases in each group: one mucinous borderline tumor and one immature teratoma in the SPLS group and one mucinous borderline tumor and one mucinous cancer in the laparotomy group. The patient's median age was not significantly different between the two groups (30 [13 to 53] years in SPLS vs. 37.5 [19 to 73] years in laparotomy,
P=0.093). There was no significant difference in median body mass index in the two groups (21.9 [17.4 to 32.1] in SPLS vs. 21.6 [17.5 to 33.1] in laparotomy,
P=0.459). There was no significant difference in the median ovarian tumor diameter in the two groups (17.0 [15 to 30] cm in SPLS vs. 20.0 [15 to 35] cm in laparotomy,
P=0.211). There were two cases wherein the surgical method was changed (9.5%) in SPLS: one patient was switched to multiport laparoscopy to expose posterior cul-de-sac and another patient was switched to laparotomy due to severe pelvic adhesion.
Table 1
Patient characteristics of SPLS and laparotomy for huge ovarian cyst (n=43)
|
Characteristics |
SPLS (n=21) |
Laparotomy (n=22) |
P-value |
|
Age (yr) |
30 (13–53) |
37.5 (19–73) |
0.093a)
|
|
Body mass index (kg/m2) |
21.9 (17.4–32.1) |
21.6 (17.5–33.116/22) |
0.459a)
|
|
Parity |
0 (0–3) |
0.5 (0–3) |
0.610a)
|
|
Ovarian tumor diameter (cm) |
17.0 (15.0–30.0) |
20.0 (15.0–35.0) |
0.211a)
|
|
Patient with pelvic adhesion |
6/21 (28.6) |
8/22 (36.4) |
0.590b)
|
|
Previous abdominal surgery |
8/21 (38.1) |
10/22 (45.5) |
0.623b)
|
|
Pathology |
|
|
0.588c)
|
|
Mucinous cystadenoma |
8 (38.1) |
9 (42.9) |
|
|
Serous cystadenoma |
1 (4.8) |
2 (9.1) |
|
|
Seromucinous cystadenoma |
2 (9.5) |
0 |
|
|
Mature cystic teratoma |
2 (9.5) |
4 (13.6) |
|
|
Endometriotic cyst |
3 (14.3) |
2 (9.1) |
|
|
Simple cyst |
1 (4.8) |
0 |
|
|
Parovarian cyst |
1 (4.8) |
0 |
|
|
Cystadenofibroma |
0 |
1 (4.5) |
|
|
Fibrothecoma |
0 |
1 (4.5) |
|
|
Inclusion cyst |
0 |
1 (4.5) |
|
|
Sclerosing stromal tumor |
1 (4.8) |
0 |
|
|
Mucinous borderline tumor |
1 (4.8) |
1 (4.5) |
|
|
Immature teratoma |
1 (4.8) |
0 |
|
|
Mucinous carcinoma |
0 |
1 (4.5) |
|
|
Operation type |
|
|
0.650b)
|
|
Salpingo-oophorectomy |
16 (76.2) |
18 (81.8) |
|
|
Cystectomy |
5 (23.8) |
4 (18.2) |
|

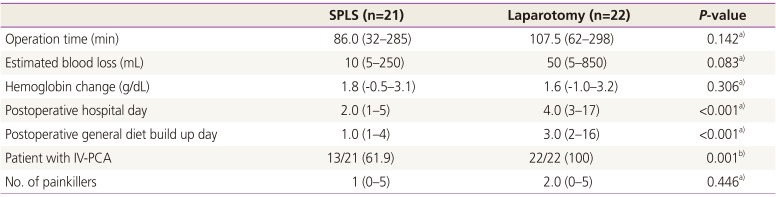
The surgical outcomes are shown in
Table 2. The median operation time was not significantly different between the two groups (median, 86.0 [32 to 285] minutes in SPLS vs. 107.5 [62 to 298] minutes in laparotomy,
P=0.142). The SPLS group had a significantly shorter hospital stay (median, 2 [1 to 5] vs. 4 [3 to 17] days;
P<0.001). The median postoperative general diet build-up days were also significantly shorter for the SPLS group than for the laparotomy group (median, 1 [1 to 4] vs. 3 [2 to 16] days;
P<0.001]. The median estimated blood loss and hemoglobin change between preoperative day and postoperative day 1 were not significantly different.
Table 2
Comparison of surgical outcomes between SPLS and laparotomy for huge ovarian cyst (n=43)
|
SPLS (n=21) |
Laparotomy (n=22) |
P-value |
|
Operation time (min) |
86.0 (32–285) |
107.5 (62–298) |
0.142a)
|
|
Estimated blood loss (mL) |
10 (5–250) |
50 (5–850) |
0.083a)
|
|
Hemoglobin change (g/dL) |
1.8 (−0.5–3.1) |
1.6 (−1.0–3.2) |
0.306a)
|
|
Postoperative hospital day |
2.0 (1–5) |
4.0 (3–17) |
<0.001a)
|
|
Postoperative general diet build up day |
1.0 (1–4) |
3.0 (2–16) |
<0.001a)
|
|
Patient with IV-PCA |
13/21 (61.9) |
22/22 (100) |
0.001b)
|
|
No. of painkillers |
1 (0–5) |
2.0 (0–5) |
0.446a)
|

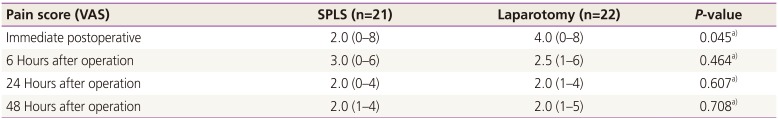
Immediate postoperative pain scores (
Table 3) were significantly different (2.0 [0 to 8] in SPLS vs. 4.0 [0 to 8] in laparotomy,
P=0.045). However, there were no statistical differences between the SPLS and laparotomy groups in pain scores at 6 hours after operation (3.0 [0 to 6] in SPLS vs. 2.5 [1 to 6] in laparotomy,
P=0.464), at 24 hours after operation (2.0 [0 to 4] in SPLS vs. 2.0 [1 to 4] in laparotomy,
P=0.607), and at 48 hours after operation (2.0 [1 to 4] in SPLS vs. 2.0 [1 to 5] in laparotomy,
P=0.708). As shown in
Table 2, patient controlled anesthesia use was in 61.9% of patients in the SPLS group and 100% of those in the laparotomy group (
P=0.001). The number of intravenous or intramuscular analgesic injections did not show a statistically significant difference between the SPLS and laparotomy groups (1.0 [0 to 5] in SPLS vs. 2.0 [0 to 5] in laparotomy,
P=0.446). There was no tumor rupture in patients who had salpingo-oophorectomy in SPLS group. There were no specific complications in the SPLS group; however, one patient in the laparotomy group had pseudomembranous colitis.
Table 3
Comparison of pain score between SPLS and laparotomy for huge ovarian cyst (n=43)
|
Pain score (VAS) |
SPLS (n=21) |
Laparotomy (n=22) |
P-value |
|
Immediate postoperative |
2.0 (0–8) |
4.0 (0–8) |
0.045a)
|
|
6 Hours after operation |
3.0 (0–6) |
2.5 (1–6) |
0.464a)
|
|
24 Hours after operation |
2.0 (0–4) |
2.0 (1–4) |
0.607a)
|
|
48 Hours after operation |
2.0 (1–4) |
2.0 (1–5) |
0.708a)
|

Go to :

Discussion
To date, there are still controversies regarding the use of SPLS for large ovarian tumors. There is no standard maximum diameter of ovarian tumor recommended for laparoscopy but it is commonly restricted to tumors under 10 cm in greatest diameter [
12] due to technical difficulties including surgical space identification in the case of a large ovarian tumor, crowding of instruments around the umbilicus, and a limited number of available instruments. A relatively long learning curve is also noted as a main difficulty of SPLS [
13].
In this study, SPLS with SW Kim's technique was successful in 90.5% of cases without any tumor spillage or conversion to multiport or laparotomy, and there were no statistical differences in length of operation time and perioperative complication. As shown in
Table 1, the SPLS group had more advantages than did the laparotomy group, including a shorter postoperative hospital stay, general diet build-up day, reduced immediate postoperative pain scores, and decreased rates of intravenous-patient controlled anesthesia. SPLS using SW Kim's technique could be a feasible and a safe procedure compared to laparotomy even in extremely huge ovarian tumors (maximal diameter of 15 to 30 cm).
A large retrospective study by Fagotti et al. [
10], looked at 125 patients who underwent SPLS for unilateral or bilateral salpingo-oophorectomies or bilateral ovarian cyst enucleations. They reported that patients who received SPLS had minimal intraoperative blood loss, no major complications, optimal postoperative pain control, and cosmetic satisfaction of the surgical wound. Yim et al. [
11] reported a retrospective case control study of 110 patients who underwent SPLS and 107 patients who underwent conventional multiport laparoscopy for adnexal surgeries. They indicated that there were no significant differences of surgical outcomes between single port and conventional multiport laparoscopy with regards to the postoperative pain score. Song et al. [
8] reported a prospective study of SPLS for large ovarian cyst (diameter ≥15 cm). They found that single port surgery for extremely large ovarian tumors is a feasible, safe and highly cosmetically satisfactory procedure. Chong et al. [
4] reported a retrospective study of 25 patients who underwent single port laparoscopy, 33 patients who underwent conventional multiport laparoscopy, and 25 patients who underwent laparotomy. It showed that the spillage rate of single port assisted extracorporeal cystectomy surgery was lower than that in conventional laparoscopy and with comparable surgical outcomes. Bedaiwy et al. [
14] reported a retrospective study of 31 patients who underwent single port laparoscopy and 57 patients who underwent conventional laparoscopy for ovarian cystectomy. These five studies indicate that single port laparoscopy for ovarian cysts is a feasible, safe, and cosmetically satisfactory procedure. However, these studies included relatively small ovarian cysts or used suction and aspiration methods in large cysts. Despite preoperative evaluations such as tumor markers and imaging studies to rule out ovarian malignancy, there is still some possibility of malignancy on the final pathological report. As shown in
Table 1, there were two cases of malignancy in each group: one borderline ovarian malignancy and one immature teratoma in the SPLS group, and one borderline ovarian malignancy and one mucinous ovarian cancer in the laparotomy group. Therefore, a large ovarian cystic tumor should be safely removed without tumor rupture. In our study, we introduced a new technique that enables safe removal of a huge ovarian tumor without rupture or aspiration.
There are several limitations of this study. First, all SPLS was mainly performed by a single surgeon, but laparotomy was performed by 4 skillful gynecology surgeons. Second, the results are based on retrospective data from a review of medical records, so there could be a selection bias. Finally, the sample sizes are relatively small in both groups. These three points may weaken the results. However, to the best of our knowledge, this study is the first report that describes the surgical technique that can remove a huge ovarian tumor without rupture or aspiration in SPLS. Furthermore, this study is the first report to make a comparison between SPLS and laparotomy for huge ovarian tumors.
In conclusion, we reported a safe and efficient method (SW Kim's technique) for removal of a huge ovarian tumor without rupture using SPLS. SPLS for huge ovarian tumors could be comparable to laparotomy regarding operation time, ovarian tumor diameter, and safety. SPLS might have more advantages in terms of postoperative hospital stay, general diet buildup days, and postoperative pain compared to laparotomy. SPLS done by experienced gynecological surgeons could be a feasible and efficacious surgery for huge ovarian tumors. However, to prove the benefits and safety of SPLS for huge ovarian tumors, multi-center randomized controlled trials in large numbers should be performed.
Go to :












 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download