Abstract
Objective
This study was done to evaluate whether perioperative propranolol (ß-blocker) in ovarian cancer patients undergoing debulking surgery reduced perioperative tumor growth induced by surgical stress.
Methods
This was a prospective randomized single institution analysis. The primary objective was to compare the changes in CA 125 level (changes between preoperation day 2 and postoperative day 7). As a study arm, patients received a low dose of propranolol 40 mg/day (4×10 mg) starting two days before surgery and 40 mg twice daily for three days following surgery.
Results
Twenty-two patients were enrolled and 16 were evaluable for efficacy. The drug was well tolerated. The mean decrease of CA 125 during the seven perioperative days was 83.1±8.9% in the propranolol group and 72.4±14.7% in the placebo group. The difference was statistically significant (P=0.044). The change of C-reactive protein, cortisol, and anxiety score (State-Trait Anxiety Inventory-X1) were not different between the two groups.
Conclusion
This preliminary result is the first to directly test the role of perioperative propranolol on tumor growth. Even with the small sample size and short term use of the drug, perioperative propranolol was effective in reducing tumor burden (as measured by CA 125) suggesting its potential benefits in decreasing perioperative tumor growth.
Epithelial ovarian cancer is the most lethal gynecological cancer. Treatment is based on initial debulking surgery followed by chemotherapy. Reoperation with secondary tumor resection has become an option for many ovarian cancer patients. Therefore, women with ovarian cancer frequently undergo several surgical procedures throughout the course of their disease.
Epidemiologic and experimental animal studies have shown that stress may alter tumor growth, and surgery is an important contributor of stress. In clinical practice, outgrowth of distant metastasis has been described following surgical removal of primary tumors [1]. This observation has been further supported by several models that have shown increased tumor growth and metastatic spread following surgery [23]. There are several potential mechanisms for the effects on tumor growth by surgical stress, including shedding of tumor cells due to physical manipulation [45], a drop in the level of antiangiogenic factors [6], local and systemic release of growth factors or cytokines [7], and suppression of cell-mediated immunity [8].
In ovarian cancer, pathways by which stress can directly regulate the growth of ovarian carcinomas in vivo have been demonstrated and adrenergic receptor beta 2 on ovarian carcinoma cells are involved in the pathways [9]. Interestingly, several retrospective cohort studies have examined the impact of beta blockers on long-term cancer outcomes [1011]. And we have examined the effects of laparotomy and extraperitoneal surgery on ovarian cancer growth and angiogenesis using orthotopic animal models [12]. The mice in the laparotomy and mastectomy groups had significantly greater tumor weight and nodules compared with anesthesia only controls. Propranolol completely blocked the effects of surgical stress on tumor growth, indicating a critical role for beta-adrenergic receptor signaling in mediating the effects of surgical stress on tumor growth.
The aim of this study was to evaluate if perioperative propranolol in ovarian cancer patients undergoing debulking surgery could reduce perioperative tumor growth induced by surgical stress.
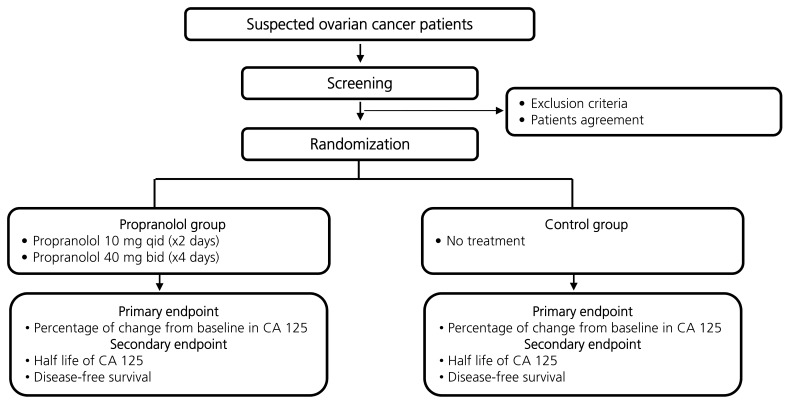
This was a prospective randomized single institution analysis of perioperative propranolol use (Fig. 1). The protocol was approved by a regional institutional review board (2009-08-056), and all patients were required to sign an informed consent form before enrollment. The primary objective was to compare the percentage of perioperative changes in the CA 125 level between the propranolol group and control group (changes between preoperation day 2 and postoperative day 7). The secondary objective was to compare the disease-free survival between the groups.
Eligible patients were those who were scheduled to undergo debulking surgery for suspected invasive epithelial ovarian cancer/primary peritoneal cancer/or fallopian tube cancer, had pretreatment CA 125 level ≥100 and Eastern Cooperative Oncology Group performance status of 2 or less, and were ≥20 years of age. Patients had to have adequate bone marrow function, renal function, hepatic function, and neurologic function. Patients had to have a pulse ≥60 beats per minute; systolic blood pressure ≥100 mmHg and diastolic blood pressure ≥60 mmHg.
We excluded patients who had received prior radiotherapy to any portion of the abdominal cavity or who had received neoadjuvant chemotherapy or targeted therapy. Patients who had significant heart failure (New York Heart Association classification ≥3), severe hyperactive airway disease (e.g., chronic obstructive pulmonary disease or asthma), or severe sinus bradycardia, or who were already on beta-blockers or in whom beta-blockers were contraindicated were also excluded.
Propranolol was administered as shown in Supplemental Fig. 1. Patients in the study arm received a low dose of propranolol 40 mg/day (4×10 mg) starting two days before surgery (immediately following the first blood draw) until the evening before the surgery. The first dose was given under supervision. On the morning of the surgery, a single tablet of propranolol 40 mg was administered and then continued twice daily for three days following surgery. Pulse and blood pressure were monitored during the first day of treatment, during the day of the surgery and on the following days. If the pulse decreased to less than 50/min or the systolic blood pressure decreased to under 90 mmHg, the dose was halved. Patients in the control arm were not treated with the study drug. The patients in the treatment arm and control arm did not know which arm they were in.
After surgery, intravenous paclitaxel (175 mg/m2) or docetaxel (75 mg/m2) plus carboplatin (area under the curve 5) combination chemotherapy were given every 3 weeks for 6 to 8 cycles. Following chemotherapy, patients were followed-up every 3 months for the first 2 years, every 6 months for the next 3 years, and every year thereafter. In some cases, magnetic resonance imaging of the pelvis or additional positron emission tomography/computed tomography and computed tomography scans were required.
The primary efficacy was measured as the change of CA 125 between pre-operation day 1 and postoperative day 7. The changes were described as 100×(CA 125 level on preoperative day 1–CA 125 level on postoperative day 7)/CA 125 level on preoperative day 1.
For assessment of secondary outcomes, cortisol, C-reactive protein, and questionnaire of stress and anxiety (State-Trait Anxiety Inventory-X1) scores were measured on preoperative day 1 and postoperative day 3. Progression-free survival was measured from the initiation of treatment to the date of the last contact or to the date of the appearance of recurrent disease.
Toxicity was graded using the National Cancer Institute Common Toxicity Criteria ver. 3.0. Each event's relationship to treatment was assessed by the treating physician and documented.
The primary hypothesis of this study was that, compared to the control group, perioperative propranolol (treatment group) would provide a much greater reduction of CA 125 (response) during the seven perioperative days. We are planning a study of the continuous response variable using an independent control group and experimental group with 1 control per experimental subject. In the pilot study, the response within each subject group was normally distributed with a standard deviation of 10%. If the true difference in the experimental and control means is 5%, we will need to study 85 experimental subjects and 85 control subjects to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) 0.9. The type I error probability associated with the test of this null hypothesis is 0.05. We expected a 10% patient loss (not evaluable), and therefore the study size was set at 94 for each group.
Nominal scale variables are shown with their frequency distribution. Differences between the groups were analyzed using the Kruskall-Wallis test and Mann-Whitney U-test for continuous variables and the chi-square test for categorical variables. A P-value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
From July 2010 to October 2011, 22 patients participated in this trial (Table 1). Ten were allocated to the propranolol group and 12 to the control group. The median age was 51 years (range, 39 to 62 years) and 47 years (range, 26 to 69 years), respectively. The majority of patients had a performance status of 0 to 1. The most common histology was serous carcinoma and there were three clear cell carcinomas, one borderline serous tumor, and two tuberculosis cases. Median preoperative CA 125 level for the propranolol group and the control group was 877 (124 to 8,655) U/mL and 490 (142 to 19,522) U/mL, respectively. Of the 22 patients enrolled, two patients were suboptimally debulked and all the others were optimally debulked. All patients received first-line chemotherapy with taxane- and platinum-based regimens.
One hundred and six doses of propranolol were administered to 10 patients in the propranolol group. Of them, none required dose reduction because of hemodynamic instability. But two doses were skipped due to nausea.
Of the 22 patients enrolled in the study, 6 were excluded from the evaluation of the response analysis; these patients comprised three with clear cell carcinoma, 2 with tuberculosis, and 1 with borderline tumor. Patients with clear cell carcinoma were excluded because this cell type shows characteristics that are distinct from serous carcinoma.
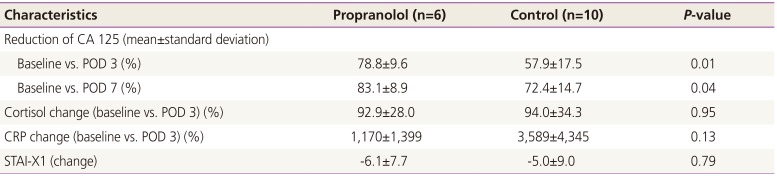
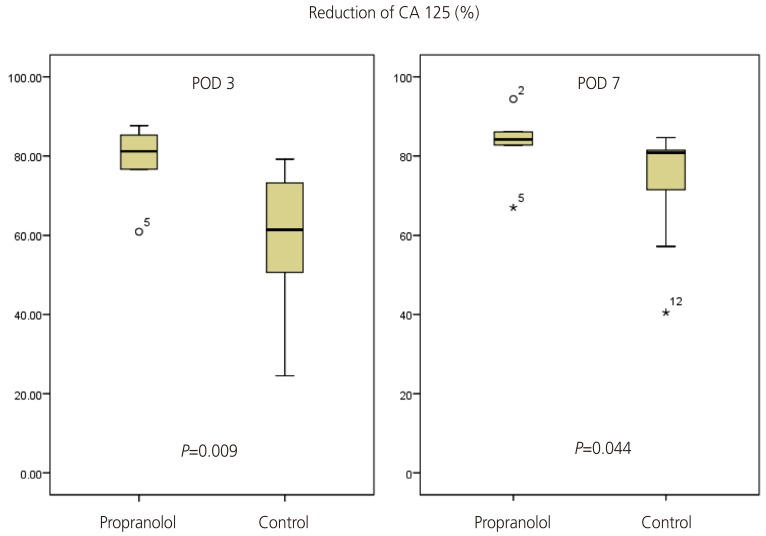
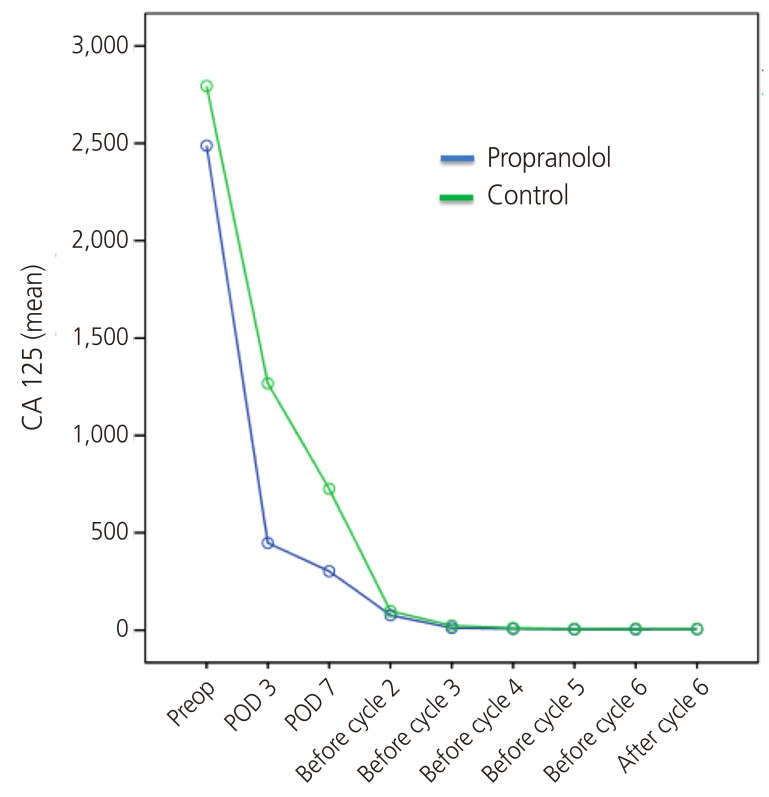
The outcomes of the 16 patients evaluated are shown in Table 2. The perioperative reduction of CA 125 was greater in the propranolol group than in the control group (Table 2, Fig. 2). The reduction of CA 125 between preoperative day 1 and postoperative day 7 was 83.1±8.9% and 72.4±14.7% in the propranolol and control groups, respectively (P=0.04). The change of CA 125 between baseline and postoperative day (POD) 3 was also statistically significant (78.8±9.6% and 57.9±17.5%, respectively, P=0.01). The mean value of CA 125 throughout the period of adjuvant chemotherapy is depicted in Fig. 3. As shown, the CA 125 level of each group was not different between chemotherapy cycle 2 to the end of chemotherapy.
For the laboratory evaluation of the stress response, we checked cortisol and C-reactive protein on preoperative day 1 and postoperative day 3 (Table 2). The perioperative change of the parameter was not different between the groups (P=0.95 and P=0.13, respectively). Similarly, the questionnaire for the evaluation of perioperative anxiety was done on preoperative day 1 and postoperative day 3. And the results did not show any significant difference between the groups (P=0.79) (Table 2).
With a median follow-up of 17 months (range, 3 to 32 months), the disease-free survival between the two groups was not different (Supplemental Fig. 2).
To the best of our knowledge, this study is the first to directly test the role of perioperative propranolol on tumor growth. The perioperative (7 day period) reduction of CA 125 was greater in patients treated with propranolol for 6 days. However, the differences disappeared 1 month following surgery. However, this result is particularly important because even with a small sample size and short-term use of propranolol, there was a significant effect as shown by CA 125. This report can be an opportunity to open a discussion on the efficacy of beta blockers. In support of this study, our previous meta-analysis showed that beta blockers had a greater positive impact on patients treated with surgery than patients not treated with surgery (data not yet published).
According to the study protocol, patients were treated with propranolol (or placebo) before surgery (2 days before surgery) and after surgery (3 days following surgery). During the study period, we decided to revise the protocol to one in which propranolol was used up to the end of chemotherapy. This decision was made because the interim analysis showed that the effect of short term propranolol (6 days) did not persist long enough to potentially enhance survival. Here we report the preliminary results of the short term use of propranolol.
In this study, the change in CA 125 was used as a surrogate marker of the tumor burden. In ovarian cancer, serial estimation of CA 125 is fairly reliable in terms of the course of the tumor and the CA 125 half time is well correlated with tumor halving time [13]. In individual patients, the reduction or increase in tumor volume correlated with the corresponding CA 125 values during chemotherapy in 85% (12 out of 14) of the patients. Mean CA 125 halving time was 44.1 days, which correlated with tumor halving time.
There are two clinical trials investigating the role of beta blockers in cancer recurrence and progression in patients with breast (NCT00502684) and colorectal (NCT 00888797) cancer undergoing surgery with curative intent. In regards to ovarian cancer, there are two phase II trials that are being conducted (NCT01308944 sponsored by Washington University and NCT01504126 sponsored by M.D. Anderson Cancer Center). In both ongoing trials, propranolol is being used to the end of chemotherapy treatment, which influenced us to revise our protocol.
As shown in our study, the brief perioperative period can be disproportionately critical for tumor growth, and hence, present an opportunity for effective intervention. Surgical excision of the primary tumor has been suspected to facilitate the progression of preexisting micrometastasis. With regard to the mechanisms involved, unavoidable damage to the tissue during surgery has been shown: (1) to increase shedding of tumor cells into the blood [5]; (2) to naturally increase levels of growth factors that also act to facilitate the growth of minimal residual disease [14]; and (3) to decrease systemic levels of antiangiogenic factors (e.g., endostatin) due to the removal of the primary tumor that induced their release [15]. And the mechanisms for these changes may be through the increase in catecholamines; they are known to act directly on malignant cells, activating several processes that are critical for tumor metastatic activity, including tumor cell proliferation [1617], extracellular matrix invasion capacity [16], resistance to apoptosis [181920], and secretion of proangiogenic factors [212223].
In addition to the role of catecholamine in these mechanisms, propranolol can be also advantageous because it is safe, accessible, and inexpensive. Beta blockers are commonly used in the treatment of cardiovascular conditions and abnormal stress responses, including social phobia and panic attacks. In addition, beta blocker, which can induce controlled hypotension, has been used to reduce bleeding and the need for blood transfusions, and provide a satisfactory bloodless surgical field [24]. Anxiety, which causes an outpouring of catecholamines, has been effectively treated with beta-adrenergic blocking drugs [2526]. Therefore, propranolol has been used to reduce perioperative anxiety and surgical tremor. Propranolol, 40 mg, administered to residents performing ocular microsurgery 1 hour prior to surgery, significantly decreased tremor and anxiety in the surgeons without untoward effects to the surgeon or the patient [27].
This study has several limitations. Firstly, all patients of the propranolol group were optimally debulked and showed higher preoperative CA 125 level than that of control group.
We cannot exclude the possibility that these two factors affect the greater reduction of CA 125 level in propranolol group. Secondly, this study did not show a significant improvement of disease-free survival in propranolol group which is clinically important. Even though, there are several studies suggest that the early drop of CA 125 level is an independent prognostic factor for survival in patients with epithelial ovarian cancer [282930].
In conclusion, although it is a preliminary and premature result, propranolol decreased CA 125 effectively during the perioperative period. Although the effect did not persist long enough, the perioperative period would theoretically present an opportunity to eradicate cancer or successfully arrest its progression. Given the limitations in this study, we consider this finding as primarily hypothesis-generating, and ongoing trials that are evaluating propranolol during the perioperative period and during chemotherapy should give more definite answers about the role of propranolol.
Acknowledgments
This study was supported by the Samsung Medical Center Clinical Research Development Program grant (CRS-110-31-1).
References
1. Peeters CF, de Waal RM, Wobbes T, Westphal JR, Ruers TJ. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer. 2006; 119:1249–1253. PMID: 16642475.

2. Wildbrett P, Oh A, Carter JJ, Schuster H, Bessler M, Jaboci CA, et al. Increased rates of pulmonary metastases following sham laparotomy compared to CO2 pneumoperitoneum and the inhibition of metastases utilizing perioperative immunomodulation and a tumor vaccine. Surg Endosc. 2002; 16:1162–1169. PMID: 11984655.

3. Lee SW, Gleason N, Blanco I, Asi ZK, Whelan RL. Higher colon cancer tumor proliferative index and lower tumor cell death rate in mice undergoing laparotomy versus insufflation. Surg Endosc. 2002; 16:36–39. PMID: 11961601.

4. Eschwege P, Dumas F, Blanchet P, Le Maire V, Benoit G, Jardin A, et al. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet. 1995; 346:1528–1530. PMID: 7491049.
5. Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000; 232:58–65. PMID: 10862196.

7. Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999; 25:231–243. PMID: 10336800.

8. Sietses C, Beelen RH, Meijer S, Cuesta MA. Immunological consequences of laparoscopic surgery, speculations on the cause and clinical implications. Langenbecks Arch Surg. 1999; 384:250–258. PMID: 10437613.

9. Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006; 12:369–375. PMID: 16428474.

10. Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011; 29:2645–2652. PMID: 21632501.

11. Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. β-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011; 20:2273–2279. PMID: 21933972.

12. Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009; 15:2695–2702. PMID: 19351748.

13. Kumar P, Rehani MM, Kumar L, Sharma R, Bhatla N, Chaudhry R, et al. Tumor marker CA-125 as an evaluator and response indicator in ovarian cancer: its quantitative correlation with tumor volume. Med Sci Monit. 2005; 11:CR84–CR89. PMID: 15668638.
14. Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer. 1999; 79:1392–1398. PMID: 10188881.

15. O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997; 88:277–285. PMID: 9008168.
16. Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011; 112:558–567. PMID: 21156980.
17. Bernabe DG, Tamae AC, Biasoli ER, Oliveira SH. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun. 2011; 25:574–583. PMID: 21187140.
18. Kerros C, Brood I, Sola B, Jauzac P, Allouche S. Reduction of cell proliferation and potentiation of Fas-induced apoptosis by the selective kappa-opioid receptor agonist U50 488 in the multiple myeloma LP-1 cells. J Neuroimmunol. 2010; 220:69–78. PMID: 20163878.

19. Roche-Nagle G, Connolly EM, Eng M, Bouchier-Hayes DJ, Harmey JH. Antimetastatic activity of a cyclooxygenase-2 inhibitor. Br J Cancer. 2004; 91:359–365. PMID: 15213717.

20. Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010; 120:1515–1523. PMID: 20389021.

21. Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006; 12:939–944. PMID: 16862152.

22. Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004; 64:2030–2038. PMID: 15026340.

23. Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009; 23:267–275. PMID: 18996182.

24. Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007; 67:1053–1076. PMID: 17488147.
25. Granville-Grossman KL, Turner P. The effect of propranolol on anxiety. Lancet. 1966; 1:788–790. PMID: 4159809.
26. Tyrer PJ, Lader MH. Effects of beta adrenergic blockade with sotalol in chronic anxiety. Clin Pharmacol Ther. 1973; 14:418–426. PMID: 4572800.

27. Elman MJ, Sugar J, Fiscella R, Deutsch TA, Noth J, Nyberg M, et al. The effect of propranolol versus placebo on resident surgical performance. Trans Am Ophthalmol Soc. 1998; 96:283–291. PMID: 10360293.
28. Riedinger JM, Eche N, Basuyau JP, Dalifard I, Hacene K, Pichon MF. Prognostic value of serum CA 125 bi-exponential decrease during first line paclitaxel/platinum chemotherapy: a French multicentric study. Gynecol Oncol. 2008; 109:194–198. PMID: 18329083.

29. Osman N, O'Leary N, Mulcahy E, Barrett N, Wallis F, Hickey K, et al. Correlation of serum CA125 with stage, grade and survival of patients with epithelial ovarian cancer at a single centre. Ir Med J. 2008; 101:245–247. PMID: 18990955.
30. Makar AP, Kristensen GB, Kaern J, Bormer OP, Abeler VM, Trope CG. Prognostic value of pre- and postoperative serum CA 125 levels in ovarian cancer: new aspects and multivariate analysis. Obstet Gynecol. 1992; 79:1002–1010. PMID: 1579296.

Supplemental Figure
Supplementary figures associated with this article can be found online at https://doi.org/10.5468/ogs.2017.60.2.170.
Supplemental Fig. 1
Schedule of propranolol administration and outcome measure. PR, pulse rate; BP, blood pressure; OP, operation; POD, postoperative day; CRP, C-reactive protein; STAI-X1, State-Trait Anxiety Inventory-X1.
Table 1
Patient characteristics at baseline
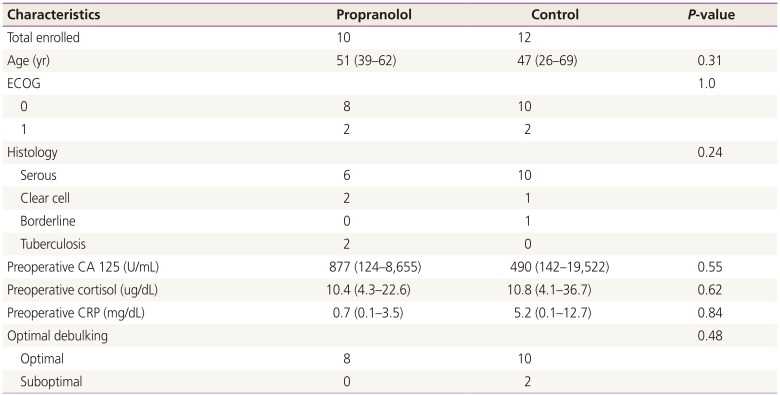
Table 2
Outcome of serum titer and anxiety score




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download