Abstract
Objective
To examine the first-trimester maternal serum placental growth factor (PlGF) and pregnancy-associated plasma protein A (PAPP-A) levels in pregnancies associated with pre-eclampsia (PE) or small-for-gestational-age (SGA) infants, and determine the predictive accuracy of PlGF and of PAPP-A for either PE or SGA infants.
Methods
This prospective, observational study included 175 pregnant women, and of these women, due to participant withdrawal or loss to follow-up, delivery data were collected from the medical records of 155 women, including 4 who had twin pregnancies. The women's maternal history was recorded, and the PlGF and PAPP-A levels at 11 to 13 gestational weeks were measured. During the second trimester, the maternal uterine artery's systolic/diastolic ratio was measured. Multiples of the median (MoM) of PlGF and PAPP-A were determined, and the associations of these values with the risk factors of SGA and PE were evaluated. Logistic regression analysis was used to determine whether PlGF and PAPP-A are useful markers for predicting SGA infants.
Results
The PAPP-A MoM level was significantly lower in women with advanced maternal age, multipara women, and women with gestational diabetes than in their counterparts. The PlGF and PAPP-A MoM levels were higher in women with a twin pregnancy than in those with a singleton pregnancy. There was a significant relationship between the maternal serum PAPP-A MoM level in the first trimester and the uterine artery systolic/diastolic ratio in the second trimester. Results of logistic regression analysis showed that low PlGF and PAPP-A MoM levels were predictors of SGA infants (odds ratio, 0.143; 95% confidence interval, 0.025 to 0.806; odds ratio, 0.191; 95% confidence interval, 0.051 to 0.718, respectively).
Pre-eclampsia (PE) is a multi-system disorder characterized by the new onset of hypertension and proteinuria or end-organ dysfunction, or both during the second half of pregnancy. Although most pregnancies with PE are associated with delivery at term or near term, with good maternal and fetal outcomes, these pregnancies have a high risk for maternal and/or fetal mortality or serious morbidity [12]. Small-for-gestational-age (SGA) infants are those that have gestational weights below the tenth percentile. Although the exact pathophysiologies of SGA infants and PE remain unknown, circulatory maladaptation characterized by defective trophoblast invasion of the maternal spiral arteries has been assumed to be associated with SGA infants and PE [3]. Impaired trophoblast invasion results in reduced feto-placental perfusion and placental dysfunction, which eventually causes poor pregnancy outcomes such as SGA infants and PE [4]. Jackson et al. reported that the placenta of SGA infants had decreased intervillous space volume, the absence of parenchyma, and few chorionic villi [5].
Currently, there is no reliable, valid, and economical screening test for SGA infants and PE. Previous studies have assessed associations between the suggested fetal weight through ultrasonography and pregnancies that resulted in SGA infants or between placenta-related proteins and pregnancies that resulted in PE or SGA infants to establish early biomarkers for SGA infants and PE [678].
The placental growth factor (PlGF) is a dimeric glycoprotein that belongs to the vascular endothelial growth factor family. It was first isolated in 1991, and it was later found to be predominantly expressed by trophoblast cells, placental villi, and human umbilical vein endothelial cells during pregnancy [9]. Studies have shown that PlGF has potent proangiogenic effects that result in early placental vascular development [8101112].
Pregnancy-associated plasma protein A (PAPP-A) is a metalloprotease that belongs to the metzincin superfamily of zinc-dependent metalloproteases. It is used as a biochemical marker of chromosomal anomalies, especially in those with Down syndrome, and it is measured during the second trimester of pregnancy. Studies have shown that low PAPP-A levels are associated with chromosomal anomalies in fetuses, and they can predict adverse pregnancy outcomes such as fetal growth restriction, spontaneous miscarriage, moderately and extremely premature births, PE, and stillbirths [131415].
An increased umbilical artery systolic-diastolic ratio has been reported to result from poorly vascularized placental villi, and it is seen in extreme cases of fetal growth restriction [16]; thus, an umbilical artery Doppler sonography is a well-known and useful adjunct for managing pregnancies complicated by fetal growth restriction.
These purposes of this study were to examine the first-trimester maternal serum placental growth factor (PlGF) and pregnancy-associated plasma protein (PAPP-A) levels in pregnancies associated with pre-eclampsia (PE) or small-for-gestational-age (SGA) infants, and to determine the predictive accuracy of PlGF and of PAPP-A for either PE or SGA infants using fetal umbilical artery systolic-diastolic ratio, which is already a well-known modality for observing placental vascularization markers.
This prospective, observational study was performed between May 1, 2012 and June 30, 2014, and it included pregnant women who received antenatal care at the National Health Insurance Service Ilsan Hospital, Goyang, Republic of Korea. One hundred seventy-five pregnant women were recruited, and blood was sampled from them during the first trimester. However, 20 women either withdrew from the study because they aborted their fetus before 25 weeks of gestation or they were lost to follow-up. Therefore, 155 women's medical records were reviewed and data were collected regarding their delivery. Among the women, 10 delivered at another hospital, and data regarding their delivery were obtained by phone. Four women had twin pregnancies, and 151 had singleton pregnancies.
All women were informed of the nature of the study, and all who agreed to participate provided written informed consent. The study was approved by the Ethics Committee of the National Health Insurance Service Ilsan Hospital (SUYON NHIMC 2012-06-005).
We assessed the associations of known risk factors for SGA infants and PE, including nulliparity, advanced maternal age, diabetes, hypertension, obesity, a history of smoking, maternal hypertension, a family history of diabetes, and twin pregnancies with PlGF and PAPP-A. Pregnancy-related data such as maternal age, gestational age, and neonatal birth weight were obtained from the patients' electronic medical records.
Maternal serum samples were collected to measure the PlGF and PAPP-A levels as part of the 11+0 to 13+6 weeks' screening program to determine the risk of fetal abnormalities, and the nuchal translucency was simultaneously assessed. Blood samples were allowed to clot between 15℃ and 22℃ for 30 to 90 minutes. Then the samples were centrifuged for 15 minutes at 300 g. The serum was collected and stored at −20℃ for up to 4 days, and then it was stored at −70℃. At the end of the study period, all samples were transferred simultaneously to a central laboratory, and they were tested for PlGF (6007-0030 DELFIA Xpress PlGF 1-2-3 kit, PerkinElmer, Turku, Finland) and PAPP-A (6003-0020 DELFIA Xpress PAPP-A Kit, PerkinElmer) using standard routine methodologies (Wallac Oy, PerkinElmer Inc., Turku, Finland).
Uterine artery Doppler examination was performed during the second trimester between 22+0 and 24+6 gestational weeks. Ultrasound scanning was performed by one expert (EHK) to reduce inter-observer variation using the Philips Ultrasound IU22 scanner (Philips, Bothell, WA, USA). The PlGF and PAPP-A levels were converted to multiples of the median (MoM) by dividing each individual result by the expected median marker of the control group at that gestational age. Maternal serum marker levels were expressed in multiples of the normal gestation-specific median based on regression analysis among the women with singleton pregnancies by combining the first-trimester results. The median level for each completed week of gestation was regressed against the median days and weighted for the number of samples tested. Gestation was derived from the crown-rump length (CRL) using a published formula (Philips Ultrasound IU22, [CRL×1.04]0.5×8.05+23.7) [17].
An infant was considered an SGA infant if the infant's birth weight was less than the tenth percentile after correcting for the reference gestational age of Koreans [18].
Differences between the groups were assessed using the Student t test to determine which factors could significantly predict PE. The odds ratio (OR) of PlGF and PAPP-A for PE and SGA infants was obtained using logistic regression analysis. Receiver operating characteristic (ROC) curves were used to assess the efficacy of clinical parameters to predict SGA infants. All P-values were two-tailed, and P-values <0.05 were considered statistically significant. All analyses were performed using the IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
One hundred seventy-five pregnant women were included in this study, and 155 women's medical record data regarding their deliveries could be collected. The women's demographic data are presented in Table 1.
The median maternal and gestational ages were 32 years and 38 weeks, respectively. Of 175 women, 103 (58.9%) were nulliparous and 72 (41.1%) were multiparous. The median PlGF and PAPP-A MoM levels were 0.92 (95% confidence interval, 0.22 to 2.85) and 1.05 (95% confidence interval, 0.10 to 4.06), respectively.
The association of the risk factors of PE and SGA infants with PlGF and PAPP-A are presented in Table 2. Table 2 includes the risk factors associated with hypertensive disorders of pregnancy (HDP) and other variable factors. The PAPP-A MoM level was significantly lower in multipara women than in primipara women and lower in women aged ≥35 years than in those aged <35 years. The PAPP-A MoM level was not associated with other HDP risk factors, and the PlGF MoM level was not associated with any HDP risk factors. However, we noted that the PlGF MoM level tended to be lower in primipara women, women aged ≥35 years, obese women, and women with maternal hypertension, and that the PAPP-A MoM level tended to be lower in women with maternal hypertension, without statistical significance. When assessing the other risk factors, we found that the PAPP-A MoM level was significantly lower in women with gestational diabetes than in those without gestational diabetes. Additionally, the PlGF MoM level was significantly higher in women with a history of smoking than in those without a history of smoking. Moreover, the PlGF and PAPP-A MoM levels were significantly higher in women with a twin pregnancy than in those with a singleton pregnancy.
In singleton pregnancies, PlGF and PAPP-A MoM levels were assessed in 18 women who delivered SGA infants (SGA group) and 133 women who delivered appropriate-for-gestational-age infants (control group); additionally, these levels were assessed in 10 women with PE (PE group) and 141 women without PE (control group). We found that the PlGF and PAPP-A MoM levels were significantly lower in the SGA group than in the corresponding control group, and the PlGF and PAPP-A MoM levels tended to be lower in the PE group than in the corresponding control group, without statistical significance (Table 3). Results of univariate logistic regression analysis of singleton pregnancies showed that low PlGF and PAPP-A MoM levels and PE were associated with SGA infants (Table 4). We found that the only significant predictors of PE are maternal age and body mass index. The PlGF and PAPP-A MoM levels could not predict PE (Table 5).
According to the the results of logistic regression analysis, the PlGF MoM level was significantly positively correlated with the PAPP-A MoM level (r=0.467, P<0.001) (Fig. 1). The PlGF MoM level in the first trimester was not significantly correlated with the uterine artery systolic-diastolic ratio in the second trimester (r=-0.179, P=0.200) (Fig. 2). However, the PAPP-A MoM level in the first trimester was significantly correlated with the uterine artery systolic-diastolic ratio in the second trimester (r=-0.029, P=0.017) (Fig. 3).
According to the ROC curves, the best cut-off points for predicting an SGA infant was 0.885 for PlGF MoM and 1.06 for PAPP-A MoM. The area under the curve (AUC) of the ROC for PAPP-A MoM (AUC, 0.728; P=0.002) was greater than that for PlGF MoM (AUC, 0.662; P=0.026) for predicting SGA infants (Fig. 4).
Recently, studies have shown that between the first and second trimesters of singleton pregnancies, changes in maternal plasma levels of angiogenic factors (e.g., vascular endothelial growth factor, PlGF, and PAPP-A) and anti-angiogenic factors (e.g., soluble fms-like tyrosine kinase-1 [sFlt-1]) and/or their ratios increase the risk of delivering SGA infants and/or developing PE [192021]. Of these factors, the present study focused on PlGF and PAPP-A because most previous studies have focused on the relationship between sFlt-1 and PlGF [1920]. Between PlGF and PAPP-A, however, no previous South Korean studies have reported on maternal PlGF, PAPP-A, and uterine artery Doppler sonogram findings.
A lower PlGF level than normal due to insufficient vascular remodeling of the spiral arteries eventually leads to reduced perfusion of the placenta, which is conceivably involved in the following adverse pregnancy outcomes: SGA, PE, HELLP (hemolysis, increased liver enzymes, and low platelets) syndrome [222324252627]. Some studies have shown that the PlGF level is low in pregnancies complicated by SGA infants and PE [67192829303132]. It has been reported that pregnancy-induced hypertension, fetal growth restriction, diabetes mellitus, and miscarriage are associated with a low maternal serum PAPP-A level at 10 to 14 weeks of gestation [333435].
Contrary to our expectation, we found that PAPP-A MoM levels were higher in primipara women and women with a BMI ≥25 kg/m2; these factors are generally regarded as risk factors for hypertensive disorders. However, the PlGF MoM levels tended to be lower, although not statistically significant, in primipara women and women with a BMI ≥25 kg/m2. One possible explanation for these findings is that PlGF is more accurate for predicting PE than PAPP-A as an independent factor, and/or PE involves more complex and various factors than delivering SGA infants. We found that the PAPP-A and PlGF MoM levels were higher in women with a twin pregnancy than in those with a singleton pregnancy. These findings are consistent with those of previous studies [3637]. It has been reported that PlGF levels are lower in women with twin pregnancies who have PE, and the PAPP-A levels increase by the same magnitude that the PlGF levels reduce, which is considered to result from placental over-compensation [3738].
The present study's findings demonstrated that in pregnancies resulting in the birth of SGA infants, the maternal serum PlGF and PAPP-A levels at 11+0 to 13+6 weeks of gestation decrease, and in normal pregnancies, there was a significant association between the serum levels of these metabolites and birth weight, which may be predetermined, which are findings consistent with the those of previous studies [67]. Our study's results are consistent with the hypothesis that impaired placentation plays a role in the pathogenesis of PE and SGA. It is noteworthy that in this study PlGF and PAPP-A were more statistically significantly associated with delivering an SGA infant than with developing PE. However, although delivering an SGA infant and developing PE were similar in that both conditions have impaired placental implantation, we found that PlGF and PAPP-A were associated with delivering an SGA infant. Additionally, in a comparison between PlGF and PAPP-A, we found that PAPP-A had a more predictive value for delivering an SGA infant. Only the PAPP-A, which is a well-known predictive factor for SGA, was associated with the second trimester umbilical artery Doppler sonogram findings [39]. The reason why PAPP-A was more predictive needs to be explored in further studies.
This study has several strengths. First, this was a prospective, observational study performed by one operator, which reduced the inter-observer variability of the ultrasound scans. Second, to our knowledge, this is the first study about the associations of PlGF and PAPP-A assessed in the first trimester in terms of PE and SGA infants in South Korea. Third, to evaluate placentation, we performed uterine artery Doppler sonography in the second trimester instead of measuring the PlGF and PAPP-A levels in the first trimester. In the future, further clinical studies on the accuracy of PlGF and PAPP-A levels as predictors of PE should be performed.
However, this study has several limitations. First, the study included a small number of women; therefore, our study only confirmed that the PlGF and PAPP-A levels in the PE group were similar to those in the control group. There is a possibility that the PE group included a smaller number of women than the SGA group in our study, and PE itself has more complex factors than SGA. Second, we did not subdivide the HDP, such as gestational hypertension, PE, eclampsia, PE superimposed on chronic hypertension, and chronic hypertension. If we could have subdivided the hypertensive disorders and had a sufficient number of patients with PE, we may have found a relationship between PE and PlGF, and PAPP-A. In conclusion, the PlGF and PAPP-A levels are potentially useful as first-trimester markers for SGA infants and some HDP. The measurement of PlGF and PAPP-A in the first trimester may be more useful in developing countries where regular antenatal care during pregnancy cannot be provided. Further studies are needed to demonstrate the different roles of PlGF and PAPP-A in placentation. Additionally, large-scale studies should be performed to further evaluate the usefulness of these markers alone and in combination with an ultrasonography examination, and other markers such as sFlt-1 should be studied further. Furthermore, early interventional trials should be designed to prevent these pregnancy complications.
Figures and Tables
Fig. 1
Relationship between the maternal serum placental growth factor (PlGF) and pregnancy-associated plasma protein A (PAPP-A). The PlGF multiples of the median (MoM) level was significantly positively correlated with the PAPP-A MoM level (r=0.467, P<0.001).
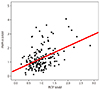
Fig. 2
Relationship between the placental growth factor (PlGF) and uterine artery systolic/diastolic (S/D) ratio. The PlGF multiples of the median (MoM) level in the first trimester was not significantly correlated with the uterine artery S/D ratio in the second trimester (r=-0.179, P=0.200).
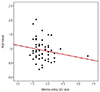
Fig. 3
Relationship between the pregnancy-associated plasma protein A (PAPP-A) and uterine artery systolic/diastolic (S/D) ratio. The PAPP-A multiples of the median (MoM) level in the first trimester was significantly associated with the uterine artery S/D ratio in the second trimester (r=-0.029, P=0.017).

Fig. 4
Receiver operating characteristics curves for the placental growth factor (PlGF) and pregnancy-associated plasma protein A (PAPP-A) multiples of the median (MoM). According to receiver operating characteristics analysis, the area under the curves (AUCs) for PAPP-A MoM (AUC, 0.728; 95% confidence interval, 0.628 to 0.828; P=0.002) and PlGF MoM (AUC, 0.662; 95% confidence interval, 0.532 to 0.792; P=0.026) were calculated.

Table 1
Demographic data of the pregnant women
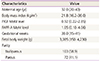
Table 2
Associations of the variables with PlGF and PAPP-A in the first trimester
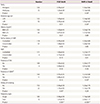
Data are presented as mean±standard deviation.
PlGF, placental growth factor; PAPP-A, pregnancy-associated plasma protein A; MoM, multiples of the median; BMI, body mass index; HiBP, high blood pressure; DM, diabetes mellitus; GDM, gestational diabetes.
a)Statistically significant; b)Diagnosed during the second trimester in a singleton pregnancy.
Table 3
Associations of SGA and PE with PlGF and PAPP-A in a singleton pregnancy

Table 4
Results of univariate logistic regression analysis for predicting a small-for-gestational-age infant in a singleton pregnancy

| Variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| PlGF MoM | 0.143 | 0.025–0.806 | 0.027a) |
| PAPP-A MoM | 0.191 | 0.051–0.718 | 0.014a) |
| Pre-eclampsia | 5.600 | 1.141–27.488 | 0.034a) |
Table 5
Results of univariate logistic regression analysis for predicting pre-eclampsia in a singleton pregnancy

| Variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| PlGF MoM | 1.502 | 0.244–9.265 | 0.661 |
| PAPP-A MoM | 1.281 | 0.540–3.037 | 0.575 |
| Age | 1.304 | 1.053–1.614 | 0.015a) |
| Body mass index | 1.230 | 1.055–1.434 | 0.008a) |
Acknowledgments
This study was supported by the National Health Insurance Service, Ilsan Hospital (no. SUYON 2013-21) .
References
1. Sibai BM, Caritis S, Hauth J. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about preeclampsia. Semin Perinatol. 2003; 27:239–246.
2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011; 25:391–403.
3. Madazli R, Budak E, Calay Z, Aksu MF. Correlation between placental bed biopsy findings, vascular cell adhesion molecule and fibronectin levels in pre-eclampsia. BJOG. 2000; 107:514–518.
4. Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001; 357:209–215.
5. Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol. 1995; 172:518–525.
6. Cowans NJ, Stamatopoulou A, Matwejew E, von Kaisenberg CS, Spencer K. First-trimester placental growth factor as a marker for hypertensive disorders and SGA. Prenat Diagn. 2010; 30:565–570.
7. Poon LC, Zaragoza E, Akolekar R, Anagnostopoulos E, Nicolaides KH. Maternal serum placental growth factor (PlGF) in small for gestational age pregnancy at 11(+0) to 13(+6) weeks of gestation. Prenat Diagn. 2008; 28:1110–1115.
8. Kasdaglis T, Aberdeen G, Turan O, Kopelman J, Atlas R, Jenkins C, et al. Placental growth factor in the first trimester: relationship with maternal factors and placental Doppler studies. Ultrasound Obstet Gynecol. 2010; 35:280–285.
9. Torry DS, Ahn H, Barnes EL, Torry RJ. Placenta growth factor: potential role in pregnancy. Am J Reprod Immunol. 1999; 41:79–85.
10. Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991; 88:9267–9271.
11. Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997; 18:657–665.
12. Vuorela P, Hatva E, Lymboussaki A, Kaipainen A, Joukov V, Persico MG, et al. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997; 56:489–494.
13. Brambati B, Macintosh MC, Teisner B, Maguiness S, Shrimanker K, Lanzani A, et al. Low maternal serum levels of pregnancy associated plasma protein A (PAPP-A) in the first trimester in association with abnormal fetal karyotype. Br J Obstet Gynaecol. 1993; 100:324–326.
14. Yaron Y, Heifetz S, Ochshorn Y, Lehavi O, Orr-Urtreger A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn. 2002; 22:778–782.
15. Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002; 87:1762–1767.
16. Todros T, Sciarrone A, Piccoli E, Guiot C, Kaufmann P, Kingdom J. Umbilical Doppler waveforms and placental villous angiogenesis in pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1999; 93:499–503.
17. Lounghna P, Chitty L, Evans T, Chudleigh T. Fetal sized and dating: charts recommended for clinical obstetric practice. Ultrasound. 2009; 17:160–166.
18. Lee JJ. Birth weight for gestational age patterns by sex, plurality, and parity in Korean population. Korean J Pediatr. 2007; 50:732–739.
19. Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008; 21:279–287.
20. Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007; 50:137–142.
21. Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008; 21:9–23.
22. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982; 142:159–167.
23. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993; 341:938–941.
24. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005; 365:785–799.
25. Curtin WM, Weinstein L. A review of HELLP syndrome. J Perinatol. 1999; 19:138–143.
26. Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001; 357:53–56.
27. Mihu D, Costin N, Mihu CM, Seicean A, Ciortea R. HELLP syndrome: a multisystemic disorder. J Gastrointestin Liver Dis. 2007; 16:419–424.
28. Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol. 2008; 199:268.
29. Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007; 196:239.
30. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004; 350:672–683.
31. Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004; 89:770–775.
32. Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001; 184:1267–1272.
33. Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007; 109:1316–1324.
34. Vatten LJ, Asvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand. 2012; 91:1388–1394.
35. Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000; 107:1265–1270.
36. Sanchez O, Llurba E, Marsal G, Dominguez C, Aulesa C, Sanchez-Duran MA, et al. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: relationship with assisted reproduction technology. Hum Reprod. 2012; 27:358–365.
37. Svirsky R, Levinsohn-Tavor O, Feldman N, Klog E, Cuckle H, Maymon R. First- and second-trimester maternal serum markers of pre-eclampsia in twin pregnancy. Ultrasound Obstet Gynecol. 2016; 47:560–564.
38. Chasen ST, Martinucci S, Perni SC, Kalish RB. First-trimester biochemistry and outcomes in twin pregnancy. J Reprod Med. 2009; 54:312–314.
39. Rochelson BL, Schulman H, Fleischer A, Farmakides G, Bracero L, Ducey J, et al. The clinical significance of Doppler umbilical artery velocimetry in the small for gestational age fetus. Am J Obstet Gynecol. 1987; 156:1223–1226.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download