Abstract
Prenatal intervention of severe fetal aortic valve stenosis by ultrasound-guided percutaneous balloon valvuloplasty has been performed to prevent the progression to hypoplastic left heart syndrome, and achieve biventricular circulation in neonates. Here we report a case of fetal aortic valvuloplasty prenatally diagnosed with aortic stenosis at 24 weeks of gestation and showed worsening features on a follow-up echocardiography. Prenatal aortic valvuloplasty was performed at 29 weeks of gestation, and was a technical success. However, fetal bradycardia sustained, and an emergency cesarean delivery was performed. To the best of our knowledge, this is the first reported case of fetal aortic valvuloplasty which was performed in Asia.
Aortic stenosis (AS) is defined as an obstruction of the left ventricular outflow caused by aortic valve narrowing. Severe AS may lead to a growth restriction of the left ventricle (LV) and aortic root, resulting in the hypoplastic left heart syndrome, which finally requires staged palliation for univentricular circulation after birth [12]. In 1991, the balloon aortic valvuloplasty was first attempted in the fetus for relieving the obstruction of the aortic valve before the evolution to hypoplastic left heart syndrome [3]. Subsequently, several groups have performed fetal aortic valvuloplasty (FAV), and on the basis of clinical reports published worldwide, FAV has demonstrated a potential benefit in achieving biventricular circulation [45]. Herein, we report our first experience of FAV in a fetus with severe AS at 29.2 weeks of gestation.
A 33-year-old multiparous woman was referred to our tertiary center with a suspected AS at 24.1 weeks of gestation. Her past medical history was unremarkable, and she had a normal Quad test. A fetal echocardiography was performed using an UGEO WS80A (Samsung Medison, Seoul, Korea) with a 2-6 MHz transabdominal probe. The four-chamber view of the heart showed a normal-sized LV with good contractility. Although a small amount of mitral regurgitation was present, there was no evidence of endocardial brightening, or left-to-right shunting flow across the foramen ovale. The left ventricular outflow tract view revealed a thickened aortic valve with post-stenotic dilatation of the ascending aorta, and the color Doppler revealed turbulent flow across the aortic valve (Fig. 1A, B). The pulsed-wave Doppler showed an increased velocity of >3 m/sec. In the aortic arch view, there was no reversed flow in the transverse aortic arch. No other abnormalities were detected, and the parents did not want to undergo fetal karyotyping.
The patient was followed up every two weeks and the ultrasonographic evaluation at 26.1 weeks revealed a newly appeared left-to-right shunt across the foramen ovale, monophasic mitral valve inflow and retrograde flow in the aortic arch (Fig. 1C). The repeated scan at 28.1 weeks showed a dilated left atrium and ventricle with aggravated status of mitral regurgitation. There was only a small amount of forward flow in the ascending aorta, and the majority of the blood filled the aorta by the reverse flow from the ductus arteriosus. This fetus was thought to be a candidate for FAV because of severe AS with well-preserved LV length, retrograde flow in the transverse aortic arch, left-to-right flow across the foramen ovale, and monophasic mitral inflow pattern; and therefore, following extensive counseling regarding the benefits and risks of FAV with maternal-fetal medicine specialists and pediatric cardiologists, the parents decided to receive the intervention.
FAV was performed under maternal spinal anesthesia at 29.2 weeks of gestation. The fetus was positioned on the anterior wall of the left chest, which was suitable for the procedure. After confirmation of the fetal position, we administered fetal intramuscular injections of atropine (20 µg/kg), vecuronium (0.2 mg/kg), and fentanyl (10 µg/kg) to prevent fetal movement, as previously described by Tworetzky et al. [6]. Under continuous ultrasound guidance, a 15-cm-long 14-gauge cannula with a stylet needle was introduced into the fetal LV at the apex (Fig. 2A). After advancing the cannula until just beneath the aortic valve, the stylet needle was removed, and a 0.014-inch guidewire with a coronary dilatation catheter (Abbott Vascular, Santa Clara, CA, USA) was introduced across the aortic valve. A 4.0-mm-sized balloon was used, and the balloon was inflated twice by a pressure gauge. Immediately following balloon dilation, we confirmed the increased amount of flow across the aortic valve, and a newly developed aortic regurgitation by color Doppler (Fig. 2B, C).
However, bradycardia and hemopericardium occurred shortly after the removal of the cannula. Despites the intracardiac injection of epinephrine (1 µg/kg) twice, bradycardia was sustained for several minutes, although hemopericardium was soon resolved. Therefore, we decided to perform an emergency cesarean delivery. A female neonate weighing 1,390 g was delivered with an Apgar score of 2 and 6 at 1 and 5 minutes. Immediately after the delivery, a resuscitation was performed, and the heart rate was recovered to over 110 beats/min within 2 minutes. The initial echocardiography indicated mild residual AS with aortic regurgitation and a moderate amount of mitral regurgitation. The serial follow-up echocardiography revealed mild AS with well-preserved LV size, and the LV contractility remained good (i.e., the LV ejection fraction was 54%). Because of the prematurity, she was discharged after 64 days of hospitalization, and at the time of drafting this report, the baby is doing well without any interventions.
To the best of our knowledge, this is the first reported case of FAV, performed in Asia. Because it was first attempted at our center, a simulation for FAV was performed with the specialists in maternal-fetal medicine and pediatric cardiology. Because all individuals had extensive experience in fetal intervention or pediatric cardiac intervention, FAV was technically possible and the outcome is promising. Of note, the time from the cannula insertion to removal did not exceed 5 minutes. From our first experience, FAV may be beneficial for severe AS, and a feasible technique when the fetus lies in an appropriate position.
The goal of FAV is to modify the physiology of the left heart in a fetus with severe AS before irreversible damage occurs to the LV, and achieve biventricular circulation, and the subsequent improvement of postnatal morbidity and mortality [7]. The indication for FAV includes the followings: severe AS with a LV length Z-score ≥-2; retrograde flow in the transverse aortic arch; left-to-right flow across the foramen ovale; monophasic mitral inflow pattern; and significant LV dysfunction [68]. A recent study reporting the natural history of 107 cases of fetal AS found that a significant proportion of fetuses who were thought to be ideal candidates for FAV, had biventricular circulation without FAV, and the authors concluded that the indication for FAV should be refined for future intervention [9]. Therefore, the patient selection is currently crucial, and only fetuses who might receive the benefits from FAV should be carefully selected.
Since the first attempt of FAV in 1991, many worldwide centers have reported FAV. The initial outcomes of FAV were disappointing because of technical problems and patient selection. With the improvement of technical skills and well-defined indication criteria, the recently published data revealed the high success rates of the procedures (range, 70% to 81%) and the improved rates of achieving biventricular circulation (range, 43% to 67%) [4510]. However, there remains a lack of data regarding long-term morbidity and mortality, and studies regarding long-term outcomes are needed to establish FAV as a standard therapeutic technique.
FAV is thought to succeed technically when the following findings are present: the catheter crosses the aortic valve and the balloon was inflated, with clear evidence of increased forward flow from LV to the aorta and/or newly developed aortic regurgitation using color Doppler confirmed by two echocardiographers [11]. In this respect, our intervention was technically successful. The techniques we used were similar to those reported previously, apart from the cannula size. Most of the reported cases used 18- or 19-gauge cannulae. However, only the 14-gauge cannula was available at the time of our intervention, leaving room for technical improvement. A larger cannula that we used might cause acute hemopericardum, leading to sustained bradycardia. Despite this complication in the present case, we achieved a biventricular circulation that remains functional. Furthermore, there were no maternal and neonatal morbidities.
Our first attempt of FAV will establish the foundation for the future clinical practice of FAV in Korea. Careful selection of patients and technically experienced skill of operators may improve the perinatal outcome of future intervention.
Figures and Tables
Fig. 1
The left ventricular outflow tract view of the heart at 24.1 weeks showing the thickening of the aortic valve (arrow in A), with turbulent flow across the aortic valve annulus on a color Doppler image (B). Follow-up evaluation at 26.1 weeks showing the retrograde flow in the aortic arch (C). aAo, ascending aorta; LV, left ventricle; dAo, descending aorta; AA, aortic arch.
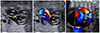
Fig. 2
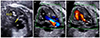
The ultrasonographic findings reveal that the cannula is located in the LV (open arrow in A), and the thickened aortic valve is demonstrated (solid arrow in A). Immediately after the balloon dilation, the ultrasonographic findings demonstrate the increased forward flow across the aortic valve (B), and a newly developed aortic regurgitation by color Doppler (C). Lt, left; Rt, right; LA, left atrium; LV, left ventricle; aAo, ascending aorta.
Acknowledgments
We would like to express special thanks to the members of our fetal intervention team, pediatric cardiologists, neonatologists, and anesthesiologists.
References
1. Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989; 25:341–343.
2. Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997; 77:205–210.
3. Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991; 65:256–258.
4. Freud LR, McElhinney DB, Marshall AC, Marx GR, Friedman KG, del Nido PJ, et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014; 130:638–645.
5. Moon-Grady AJ, Morris SA, Belfort M, Chmait R, Dangel J, Devlieger R, et al. International fetal cardiac intervention registry: a worldwide collaborative description and preliminary outcomes. J Am Coll Cardiol. 2015; 66:388–399.
6. Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome. Candidate selection, technique, and results of successful intervention. Circulation. 2004; 110:2125–2131.
7. Schidlow DN, Tworetzky W, Wilkins-Haug LE. Percutaneous fetal cardiac interventions for structural heart disease. Am J Perinatol. 2014; 31:629–636.
8. Makikallio K, McElhinney DB, Levine JC, Marx GR, Colan SD, Marshall AC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006; 113:1401–1405.
9. Gardiner HM, Kovacevic A, Tulzer G, Sarkola T, Herberg U, Dangel J, et al. Natural history of 107 cases of fetal aortic stenosis from a European multicenter retrospective study. Ultrasound Obstet Gynecol. 2016; 48:373–381.
10. Arzt W, Wertaschnigg D, Veit I, Klement F, Gitter R, Tulzer G. Intrauterine aortic valvuloplasty in fetuses with critical aortic stenosis: experience and results of 24 procedures. Ultrasound Obstet Gynecol. 2011; 37:689–695.
11. McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009; 120:1482–1490.
Supplemental Video
Supplementary videos associated with this article can be found online at https://doi.org/10.5468/ogs.2017.60.1.106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download