Abstract
Vaginal intraepithelial neoplasia III, is a relatively rare disease. Consequently standard treatments for this disease were not established until recently. Although several convenient methods, such as laser ablation, 5-fluorouracil topical injection, and radiation therapy, have been applied for treating these lesions, surgical treatments, including vaginectomy, have not yet been attempted, as they would likely be accompanied by technical difficulties and various complications. Herein, we report a case of refractory vaginal intraepithelial neoplasia III in the vaginal vault that was successfully treated with a total vaginectomy.
Following treatment for microinvasive cervical cancer, continuous follow-up tests on the vagina should be conducted, because there is a possibility that cervical cancer could recur in this location [1]. If carcinoma in situ (CIS) of the vagina or vaginal intraepithelial neoplasia (VAIN) III is found to persist in a patient who has had a hysterectomy, then nonsurgical treatments, such as carbon dioxide laser ablation, 5-fluorouracil (5-FU) topical injection, radiation therapy, and electrofulguration can be tried initially. However, surgical removal of the affected lesions can also be considered if nonsurgical treatments fail to achieve a cure. Surgical treatments, including vaginectomy procedures, have rarely been attempted due to surgical difficulties, wound complications, and concerns about quality of life. In the present study, total vaginectomy will be discussed as a treatment option for refractory VAIN III in the short vagina of a patient who had previously underwent a hysterectomy due to microinvasive cervical cancer.
A 60-year-old gravida 3, para 2 female patient, who became menopausal at 52 years old, visited the outpatient department for constant VAIN III. She had been diagnosed with cervical cancer Ia1 by conization. Pathologically, the biopsied cervical tissue was 3×2 cm in size with 2-mm depth lesion and showed no lymphovascular invasion or involvement of the resection margin from the in situ squamous cell carcinoma diagnosed 5 years earlier. She underwent a laparoscopically-assisted vaginal hysterectomy and bilateral salpingo-oophorectomy two months later. From the operation, the pathological finding was squamous cell carcinoma in situ without residual tumor. Six months after the operation, she underwent a punch biopsy and HPV DNA test at a follow-up appointment. The results were VAIN III and she was positive for an HPV 16 DNA infection. Thus, the patient underwent laser vaporization and a topical injection of 5-FU on the affected area and a second punch biopsy and human papillomavirus (HPV) DNA test six months later. The results were the same. She had a total of eight brachytherapy treatments on her entire vagina. However, VAIN III continued to appear at follow-up examination seven months later. Finally, she visited the outpatient department of our hospital for further evaluation and management.
Tumor marker and imaging tests (abdominopelvic computed tomography [CT], magnetic resonance imaging [MRI], and positron emission tomography [PET]) were performed. The squamous cell carcinoma antigen (SCC) level was 0.8 ng/mL (normal range: <2 ng/mL), and a small round cystic lesion in the vaginal stump was found on an abdominopelvic CT. A possible protein-rich Gartner's duct cyst at the left lateral aspect of the vagina was found on the MRI, but no grossly definite tumor lesion in the vagina or sign of recurrence was shown on the PET.
First, electrocauterization was performed on the stump and the vagina of the patient. The results of a Pap smear four months later showed reactive cellular changes. The next Pap smear was carried out after 3 months, and it showed atypical squamous cells of undetermined significance; therefore, a punch biopsy was performed, the results of which were VAIN III. As disease recurrence was suspected, PET was performed, and a focal hypermetabolic lesion was found around the vaginal orifice area, while the SCC level was within the normal limit (1.1 ng/mL). The follow-up MRI also showed a slightly increase in the size of the round peripheral-enhancing lesion in the left vaginal stump. Therefore, we decided to carry out the operation due to suspicion of cancer recurrence. As the cervix of the patient was very shortened (approximately 3 cm in length) due to previous radiation therapy, we decided to perform a total vaginectomy, parametrectomy, and pelvic lymph node dissection. The plastic surgery department was consulted for reconstruction of the vagina as a collaborative practice before surgery, but the patient refused. Double-J stents were inserted into both sides of ureter before the surgery to prevent ureter injury.
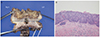
A total vaginectomy with a parametrectomy and bilateral pelvic lymph node dissection were performed resulting in the removal of a 7.5×4×1.5-cm section of vaginal tissue (Fig. 1). To dissect the pelvic area effectively, the vaginal stump was elevated and palpated by using an intravaginal elevator. The vaginal canal and paravaginal tissue were dissected meticulously until the pelvic floor was encountered. The total vaginal canal and paravaginal tissues were removed with en bloc manner by vaginal approach. The pathological result was VAIN III, a 2-cm tumor with severe ulceration, but no lymphovascular or perineural invasion (Fig. 2). The resection margin was negative for malignancy, and both the parametrium and lymph nodes were also negative for malignancy. Peritoneal fluid cytology showed benign atypia. There were no acute complications or abnormal findings on the CT seven days after surgery. Both double-J stents were removed 3 weeks later after cystoscopy was performed. During 13 months of follow-up, there were no symptoms of complications or signs of disease recurrence. Cytology and HPV DNA tests were performed every 3 months, and an abdominopelvic CT and serum SCC were checked every 6 months during the follow-up period. There was no evidence of local tumor recurrence. Future ongoing observation is planned.
Excluding partial upper vaginectomy or pelvic exenteration, this case is the first in the literature that successfully applied total vaginectomy to refractory VAIN III or suspected cancer recurrence of the vagina. There are several treatment options for VAIN III. The treatment can be decided according to the location and number of lesions, length of vagina, treatment history of radiation and surgery, and the physician's and patient's characteristics and preferences.
Laser CO2 vaporization and 5-FU topical injection are frequently used for multifocal VAIN areas that are difficult to treat [2]. Laser vaporization is most commonly used and can control the depth and width of the lesion easily, with rapid recovery after treatment [3]. However, laser vaporization shows a high recurrence rate. Dodge et al. [4] reported that only 61% of patients treated with laser vaporization were cured, regardless of the VAIN grade, however, the recurrence rate was 39%. Due to the fact that a VAIN III lesion can be located in inaccessible sites, localized therapy, such as CO2 laser vaporization, is likely to leave parts of the lesion remaining in the vagina [2].
5-FU topical injection has advantages, such as a low risk of damage and anatomic defects, as well as usefulness for multifocal or extensive lesions [5]. However, Dodge et al. [4] also reported a cure rate of 5-FU topical injection of just 40%. Moreover, complications, such as vaginal irritation, can easily occur because the anticancer agent is injected into the vagina.
Radiation therapy is also known to be an effective treatment for VAIN III. Song et al. [6] reported on 34 patients who received radiation therapy due to VAIN III after a hysterectomy. A high cure rate of 88.2% was observed with a high dose of brachytherapy. However, approximately half of the 34 patients with VAIN showed toxicity of the urinary tract, vagina, and rectum, which required continuous treatment and observation. In addition, radiation-induced cancer is another major concern associated with radiation therapy [7].
Physicians can consider surgical treatments even if VAIN III recurs after these nonsurgical treatments are performed. However, due to the fact that the vagina is located close to the bladder and rectum, surgery is considered very difficult, and location-related complications, such as vesicovaginal fistula, cystitis, and neurogenic bladder, can lead to a reluctance to perform surgery; moreover, if patients have undergone surgery or radiation treatment previously, surgery becomes more difficult [8].
However, Diakomanolis et al. [9] made a comparison between laser ablation and upper vaginectomy as treatments for VAIN. A cure rate of 80% was observed in vaginectomy patients, while a cure rate of 68% was observed in laser ablation patients. The rates of recurrence were 20% in vaginectomy patients and 32% in laser patients. Hoffman et al. [10] reported on 23 patients who had undergone upper vaginectomy due to VAIN. The cure rate was 82%, and the recurrence rate was 17%. In addition, Indermaur et al. [11] reported on 105 VAIN patients who had undergone upper vaginectomy, finding an 88% cure rate, although the follow-up loss was relatively high. The complications of vaginectomy in their study were cystotomy (n=5), cellulitis (n=1), and vaginal hemorrhage (n=1). Nevertheless, all patients recovered through proper treatment afterward. In their report, complications from the vaginectomy were not of concern due to the skillful surgeon. According to the previous three studies, the upper vaginectomy can be a treatment of choice for VAIN III in patients who have undergone a hysterectomy. Patient selection and a skilled surgeon are very important factors in successful treatment [91011].
Various nonsurgical treatments for VAIN III repeatedly failed in our patient. Moreover, she suffered from a very short vagina due to previous treatments. Therefore, we decided to resect the entire vaginal canal after comprehensive counseling. A total vaginectomy was performed successfully without any complications, including urinary tract dysfunction and wound problems. No evidence of disease recurrence was observed during the 13 months post-operation.
In conclusion, vaginectomy can be carried out in VAIN III for patients who have not been cured by nonsurgical treatments, which include laser vaporization, 5-FU topical injection, radiation therapy, and electrofulguration. Although a partial vaginectomy is performed more commonly than a total vaginectomy, a total vaginectomy can also be performed as necessary, such as in instances of a short vagina. The complications of the vaginectomy could be decreased with adequate patient selection and meticulous surgical procedure.
Figures and Tables
References
1. Li Z, Barron S, Hong W, Karunamurthy A, Zhao C. Surveillance for recurrent cancers and vaginal epithelial lesions in patients with invasive cervical cancer after hysterectomy: are vaginal cytology and high-risk human papillomavirus testing useful? Am J Clin Pathol. 2013; 140:708–714.
2. Frega A, Sopracordevole F, Assorgi C, Lombardi D, DE Sanctis V, Catalano A, et al. Vaginal intraepithelial neoplasia: a therapeutical dilemma. Anticancer Res. 2013; 33:29–38.
3. Hatch KD. A3. Vaginal intraepithelial neoplasia (VAIN). Int J Gynecol Obstet. 2006; 94:S40–S43.
4. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001; 83:363–369.
5. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997; 176(1 Pt 1):93–99.
6. Song JH, Lee JH, Lee JH, Park JS, Hong SH, Jang HS, et al. High-dose-rate brachytherapy for the treatment of vaginal intraepithelial neoplasia. Cancer Res Treat. 2014; 46:74–80.
7. Ogino I, Kitamura T, Okajima H, Matsubara S. High-dose-rate intracavitary brachytherapy in the management of cervical and vaginal intraepithelial neoplasia. Int J Radiat Oncol Biol Phys. 1998; 40:881–887.
8. Holschneider CH, Berek JS. Valvar cancer. In : Berek JS, Novak E, editors. Berek & Novak's gynecology. 14th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2007. p. 561–602.
9. Diakomanolis E, Rodolakis A, Boulgaris Z, Blachos G, Michalas S. Treatment of vaginal intraepithelial neoplasia with laser ablation and upper vaginectomy. Gynecol Obstet Invest. 2002; 54:17–20.
10. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992; 166(1 Pt 1):30–33.
11. Indermaur MD, Martino MA, Fiorica JV, Roberts WS, Hoffman MS. Upper vaginectomy for the treatment of vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 2005; 193:577–580.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download