Abstract
A 35-year-old pregnant woman visited our department and had been treated with 100 µg of daily oral levothyroxine for hypothyroidism. An ultrasonography screening was performed at 25 weeks gestation and revealed a fetal goiter and an increased amniotic fluid volume. Fetal hypothyroidism was confirmed by cordocentesis and amniotic hormone levels at 26 weeks gestation. We treated the mother with 200 µg of daily oral levothyroxine to optimize the transplacental transfer. A total of four intra-amniotic injections of levothyroxine were administered, resulting in progressive reduction in the fetal thyroid volume of goiter as measured by 3D ultrasonography and increased amniotic fluid volume. Following birth, neonatal serum thyroid stimulating hormone level was within the normal range, but free T4 was reduced. Based on this case, we suggest that monitoring amniotic fluid thyroid hormone concentration and intra-amniotic levothyroxine injection can be used to reduce the thyroid volume of goiters and to prevent polyhydramnios.
Fetal goiters are associated with hyperthyroidism, hypothyroidism, or a euthyroid state. Congenital hypothyroidism occurs in approximately 1 of every 4 to 5,000 live births. Fetal goitrous hypothyroidism is extremely rare but more common than fetal hyperthyroid and euthyroid states [1]. Congenital hypothyroidism can be caused by thyroid dysgenesis, dyshormonogenesis, transplacental thyroid stimulating hormone (TSH) receptor blocking antibodies, or maternal antithyroid medications.
Fetal goitrous hypothyroidism is diagnosed in utero based on sonography and TSH and free thyroxine levels on cordocentesis. Cordocentesis is the gold standard method for confirming fetal thyroid hormone levels for a fetal thyroid diagnosis [23]. In utero, large goiters in the fetus may cause polyhydramnios due to esophageal compression, tracheal compression, and neck hyperextension, possibly resulting in asphyxia and death [2].
If fetal thyroid dysfunction is undiagnosed in utero, it may significantly increase fetal and neonatal morbidity and mortality [4]. Untreated fetal hypothyroidism may result in mental retardation and perceptual-motor, visual-spatial, and language problems [5]. Although postnatal treatment is possible, the severity of congenital hypothyroidism is related to the degree of intellectual development in affected fetuses [67]. Therefore, in utero early diagnosis and treatment of fetal hypothyroidism are important.
A 35-year-old multigravida pregnant woman visited our department at 13 weeks of gestation. Her first baby was delivered vaginally with no complications during the antenatal or postnatal period. The woman had been diagnosed with hyperthyroidism and underwent radioactive iodine uptake treatment at 12 years of age. She was treated for hypothyroidism after radioactive iodine uptake and took 100 µg of oral levothyroxine daily. Sonography screening for routine check-up was performed at 25 weeks gestation and revealed a 21×14 (right lobe), 26×22 (left lobe)-mm-sized fetal neck mass (Fig. 1A). The location and echotexture were suggestive of a fetal goiter. On color Doppler imaging, the area around the goiter showed increased peripheral vascularity. However, the amniotic single pocket was 6.8 cm, and the amniotic fluid volume was relatively high. No other abnormality was noted in the fetus. Fetal hypothyroidism was confirmed by cordocentesis at 26 weeks gestation. The results of fetal blood sampling were as follows: TSH 390 U/mL (reference range, 0.5 to 4.5) and free T4 6.7 pmol/L (reference range, 10 to 28).The level of amniotic fluid hormone was checked at 26 weeks for reference of levothyroxine injection (Table 1). Maternal thyroid function test was performed at 26 weeks gestation. Serum TSH was 3.8 U/mL (reference range, 0.5 to 4.5), free T4 was 12.1 pmol/L (reference range, 10 to 28), T3 was 113 ng/dL (reference range, 97 to 170), thyrotropin binding inhibitor immunoglobulin/thyroid stimulating immunoglobulin was 16%, anti-thyroglobulin antibody was 2.6 U/mL (reference range, 0 to 0.3), and antimicrosome antibody was 28 U/mL (reference range, 0 to 0.3).
The maternal dose of levothyroxin was increased to 200 µg/day from 100 µg/day at 26 weeks gestation. However, even with treatment, the fetal goiter size did not change on ultrasonography. Therefore at 30+2 weeks, in-utero treatment was initiated with 200 µg of levothyroxine injected into the amniotic sac. The fetal goiter mass size had not changed. The fetus was assessed weekly by determining thyroid hormone levels via amniocentesis. At 31+1, 35+6, and 36+5 weeks, 400 µg levothyroxine was injected into the amniotic sac. At 32+1, 33+6, and 34+5 weeks, we did not treat with a levothyroxine injection because the amniotic TSH gradually decreased, and the free T4 was within the lower normal range (Table 1).
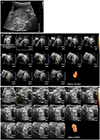
We measured the change in thyroid size by 3D ultrasonography. This was the first application of 3D ultrasonography to assess changes in fetal thyroid size in Korea. The thyroid volume was 6.6 cm3 at 29 weeks gestation (Fig. 1B) and 4.9 cm3 at 37 weeks gestation (Fig. 1C). The patient was admitted to the delivery room due to labor pain at 38+2 weeks. She delivered a healthy girl who weighed 2,495 g, with Apgar scores 8 at 1 minute and 9 at 5 minutes. The infant did not have any breathing difficulties and no palpable mass in the anterior neck. The infant was not intubated.
At 3 days after birth, thyroid ultrasonography and thyroid function tests were performed. The echogenicity of the thyroid gland was normal without definite focal lesions. No abnormally enlarged lymph node was observed on either anterior side of the neck. Neonatal serum TSH was 11.84 ulU/mL and was within the normal range (1 to 4 days; reference range, 10.0 to 18.0), but the free T4 17.9 pmol/L was low (1 to 4 days; reference range, 28.4 to 68.4). The infant and mother were discharged on the third day after delivery without any complications. During two months of follow-up, the baby was treated with daily oral thyroxine. Currently, three years after birth, the baby has not had any neurodevelopmental problems.
Fetal goiter is defined as a circumference or diameter above the 95th percentile for fetal thyroid gland size. The fetal thyroid gland can be accurately assessed by serial ultrasound from 20 to 36 weeks [8]. Although we identified fetal goiter with sonography, we could not make a specific diagnosis until after birth. In many cases, cordocentesis enables a more accurate diagnosis [2]. Serial fetal blood sampling is necessary to accurately determine fetal thyroid hormone concentration. However, this procedure has a high risk of adverse events such as fetal cord blood vessel contraction and bleeding, cord hematoma, feto-maternal bleeding, premature rupture of membranes, preterm birth, sepsis, and fetal death. The correlations between amniotic fluid and fetal serum levels of TSH, T3, and free T4 remain unclear [9]. However, amniocentesis is used to assess fetal thyroid function because it is easier to perform and safer than cordocentesis.
Therefore, we confirmed fetal hypothyroidism through cordocentesis and serially evaluated thyroid hormone levels through amniocentesis, because measuring thyroid hormone levels in amniotic fluid is reflecting fetal rather than maternal thyroid function [10]. In our case, when cordocentesis was performed at 26 weeks gestation, the TSH level was significantly elevated compared with the amniotic TSH concentration. Although amniotic fluid TSH value clearly underestimated the degree of hypothyroidism, therapy was adjusted based on the results of the amniocentesis.
In 1980, the first intra-amniotic levothyroxine administration was performed as an in utero fetal goitrous hypothyroidism treatment. Until now, intra-amniotic replacement therapy has been used as the standard treatment in fetal goitrous hypothyroidism.
In our case, intra-amniotic therapy was started at 30 weeks gestation; as soon as the intra-amniotic levothyroxine was administered, the amniotic TSH level decreased. Therefore, we found that TSH level rapidly responded to intra-uterine treatment [11]. However, amniotic free T4 level was similar to the level before intra-amniotic levothyroxine therapy, whereas the T3 level was increased after serial levothyroxine administration.
The number of cases treated in utero is currently too small to outline clear recommendations for optimal doses of free T4. Effective doses have varied from 250 µg levothyroxine in a single intra-amniotic injection to more than 3,000 g in repeated injections. There were no complications at birth from goiter size, in any of the cases. In our case, in utero treatment was initiated when 200 µg of levothyroxine was injected into the amniotic sac, and three additional intra-amniotic injections of 400 µg levothyroxine were administered between 30 and 37 weeks gestation. Intra-amniotic levothyroxine therapy was feasible and induced no adverse events.
The prenatal thyroid state is determined mainly by indirect assessment of fetal size, fetal heart rate, and thyroid goiter. Because non-invasive procedures are preferable for treatments, three-dimensional sonography can be used to evaluate and manage fetal goiters [12].
The first report of three-dimensional sonography of fetal goiter was introduced in 2005. This technique allowed measurement of fetal thyroid volume and blood flow and monitoring of the increase in size prior to therapy, in addition to regression after intra-amniotic thyroxine injections [1213]. In our case, 3D ultrasonography indicated progressive reduction in volume of the goiter; this is the first case to use 3D ultrasonography to assess fetal thyroid volume in Korea.
It is difficult to determine if a fetus will have a goiter, therefore, it is important to use non-invasive techniques, such as continuous thyroid sonographic evaluation, to assess mothers with thyroid diseases, regardless of current treatment regimen. Additionally, specialists can be trained to perform intraamniotic levothyroxine injections or cordocentesis in order to assess, diagnose, and treat similar conditions.
In conclusion, monitoring of amniotic fluid hormonal concentrations and intra-amniotic levothyroxine injection were useful for reducing the volume of the goiter and to prevent polyhydramnios. And 3D ultrasonography can be a good alternative tool for assessing a change of fetal goiter.
Figures and Tables
Fig. 1
Measurement of fetal goiter volume change using 2D and 3D ultrasonography. (A) Fetal goiter size at 25 weeks using 2D ultrasonography. Right (Rt) lobe (21×14) and left (Lt) lobe (26×22). (B) Fetal thyroid volume (6.6 cm3) at 29 weeks' gestation using 3D ultrasonography. (C) Fetal thyroid volume (4.9 cm3) at 37 weeks' gestation using 3D ultrasonography.
References
1. Dussault JH, Coulombe P, Laberge C, Letarte J, Guyda H, Khoury K. Preliminary report on a mass screening program for neonatal hypothyroidism. J Pediatr. 1975; 86:670–674.
2. Davidson KM, Richards DS, Schatz DA, Fisher DA. Successful in utero treatment of fetal goiter and hypothyroidism. N Engl J Med. 1991; 324:543–546.
3. Perelman AH, Johnson RL, Clemons RD, Finberg HJ, Clewell WH, Trujillo L. Intrauterine diagnosis and treatment of fetal goitrous hypothyroidism. J Clin Endocrinol Metab. 1990; 71:618–621.
4. Becks GP, Burrow GN. Thyroid disease and pregnancy. Med Clin North Am. 1991; 75:121–150.
5. Bernardes LS, Ruano R, Sapienza AD, Maganha CA, Zugaib M. Nomograms of fetal thyroid measurements estimated by 2-dimensional sonography. J Clin Ultrasound. 2008; 36:193–199.
6. Glorieux J, Dussault J, Van Vliet G. Intellectual development at age 12 years of children with congenital hypothyroidism diagnosed by neonatal screening. J Pediatr. 1992; 121:581–584.
7. Kooistra L, Laane C, Vulsma T, Schellekens JM, van der Meere JJ, Kalverboer AF. Motor and cognitive development in children with congenital hypothyroidism: a long-term evaluation of the effects of neonatal treatment. J Pediatr. 1994; 124:903–909.
8. Ho SS, Metreweli C. Normal fetal thyroid volume. Ultrasound Obstet Gynecol. 1998; 11:118–122.
9. Francois A, Hindryckx A, Vandecruys H, Van Schoubroeck D, Vanhole C, Allegaert K, et al. Fetal treatment for early dyshormonogenetic goiter. Prenat Diagn. 2009; 29:543–545.
10. Hanono A, Shah B, David R, Buterman I, Roshan D, Shah S, et al. Antenatal treatment of fetal goiter: a therapeutic challenge. J Matern Fetal Neonatal Med. 2009; 22:76–80.
11. Corral E, Reascos M, Preiss Y, Rompel SM, Sepulveda W. Treatment of fetal goitrous hypothyroidism: value of direct intramuscular L-thyroxine therapy. Prenat Diagn. 2010; 30:899–901.
12. Marin RC, Bello-Munoz JC, Martinez GV, Martinez SA, Moratonas EC, Roura LC. Use of 3-dimensional sonography for prenatal evaluation and follow-up of fetal goitrous hypothyroidism. J Ultrasound Med. 2010; 29:1339–1343.
13. Nath CA, Oyelese Y, Yeo L, Chavez M, Kontopoulos EV, Giannina G, et al. Three-dimensional sonography in the evaluation and management of fetal goiter. Ultrasound Obstet Gynecol. 2005; 25:312–314.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download