Abstract
The incidence of uterine arteriovenous malformation (AVM) is rare. However, it is clinically significant in that it can cause life-threatening vaginal bleeding. We report a case of a large uterine AVM with positive serum beta-human chorionic gonadotropin. A presumptive diagnosis was made; a uterine AVM accompanied by, early pregnancy or retained product of conception. Because this uterine AVM was extensive, transcatheter arterial embolization of both uterine arteries and extra-uterine feeding arteries was performed. Three months after undergoing transcatheter arterial embolization, complete resolution of the uterine AVM was confirmed without major complication.
Although uterine arteriovenous malformation (AVM) is known in gynecologic practice to be a rare disease, it seems to occur more frequently than has been reported in the literature [123]. These lesions consist of abnormal vascular proliferations and multiple variable-sized arteriovenous communications between intramural branches of the uterine artery and the myometrial venous plexus [23].
Here we present a case of a large uterine AVM accompanied by positive serum beta-human chorionic gonadotropin (β-hCG) with excessive vaginal bleeding. To our knowledge, it is the largest diameter of AVMs in comparison to published case reports in Korea. The patient's condition, diagnosis and treatment are reviewed in the hope that management of uterine AVM, thereby, can be rendered more consistently effective and safe.
A 38-year-old woman, gravida 4, para 2, was referred to our hospital for a suspicious hypervascular uterine mass. She presented prolonged and profuse vaginal bleeding for 2 months. Her previous menstrual cycles had been regular and her last menstruation was 3 months previously. She had a history of two cesarean sections and two artificial dilation and curettage. On observation of the uterine cavity at the time of the second cesarean section 5 years earlier, there were no abnormal findings. Also, there was no abnormal vaginal bleeding during and after the last dilation and curettage in 2012. Her medical histories were unremarkable. Her vital signs were stable. The initial hemoglobin level, 12.4 g/dL, decreased to 10.9 g/dL after her hospitalization. The serum β-hCG level was slightly elevated, to 496 mIU/mL.
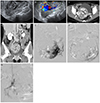
Transvaginal ultrasonography (US) revealed a large inhomogeneous complex-echoic mass in the uterus. There was no gestational sac in the uterus (Fig. 1A). Subsequent abdominal computed tomography (CT) scans narrowed down the location of the lesion, measuring approximately 8 cm, to the anterior myometrium and endometrial cavity. The lesion manifested numerous irregular, tubular, anechoic structures. These structures generated a multi-directional and turbulent flow on color Doppler US (Fig. 1B). Markedly engorged parametrial and ovarian veins, with early enhancement in the arterial phase, were evident on contrast-enhanced abdominal CT (Fig. 1C, D). Based on the clinical and radiological findings, a presumptive diagnosis was made; uterine AVM accompanied by, early pregnancy or retained products of conception. From clinical experience, gestational trophoblastic disease (GTD) cannot be ruled out definitively. Further diagnosis and treatment options were discussed with the patient. Neither curettage nor surgical biopsy, including hysterectomy, was suitable for this patient due to the possibility of fatal vaginal bleeding. For this reason, an alternative procedure transcatheter arterial embolization (TAE) was applied. The patient received 3 times of 50-mg methotrexate and 5-mg leucovorin prophylactically via intramuscular injection.
Accessing the right common femoral artery, a pelvic and both uterine artery angiograms revealed a large uterine AVM (Fig. 1E). The lesion was supplied by both, hypertrophied uterine arteries. Gelatin sponge pledgets (Cutanplast, Mascia Brunelli, Milanom, Italy) were introduced into both uterine arteries until stasis of flow was achieved. This was followed by proximal embolization with microcoils (Nester, Cook, Bloomington, IN, USA) to enhance occlusion. Transvaginal US and abdominal CT performed the next day revealed that the large vascular uterine mass remained unchanged. Five days later, the patient was referred for repeat TAE. An abdominal aortogram and super-selective angiograms showed residual staining of the uterine AVM, with fine feeders arising from the anterior division of both internal iliac arteries and both the two round ligament arteries arising from the inferior epigastric artery (Fig. 1F, G). These extra-uterine feeders also were embolized with gelatin sponge pledgets and polyvinyl alcohol particles (355-500 µm; Contour, Boston Scientific, Cork, Ireland). A post-embolization pelvic angiogram showed non-opacification of the uterine AVM. Transvaginal US performed immediately after the repeat TAE demonstrated that Color Doppler had not detected the vascularity within the lesion.
After TAE, there were no procedure-related major complications and no further vaginal bleeding occurred. Regular follow-up transvaginal US revealed that the lesion had been gradually shrinking. Three months after undergoing TAE, complete resolution of the uterine AVM was finally confirmed: the uterus and both ovaries were normal (Fig. 2). One month post-TAE, the serum β-hCG became negative, and normal menstrual cycles resumed.
The true incidence of uterine AVM is unknown. According to a literature review, uterine AVMs are most frequently encountered in women of reproductive age (mean age, 30±9.1 years); in fact, 96% of patients are premenopausal women [45]. In most cases, uterine AVM is acquired, and has been reported as a consequence of previous pelvic surgery, curettage, treatment by intra-uterine device, delivery, pathologic pregnancy-related events such as GTD, gynecologic malignancy, infection, and maternal exposure to diethylstilbestrol [56].
Uterine AVM lesions are composed of a proliferation of multiple, wide, tortuous arteriovenous channels [5]. This is confirmed by angiographic findings, which typically show a complex tangle of vessels supplied by enlarged feeding arteries associated with rapid early venous filling and stasis of contrast medium within the abnormal vasculature [7]. These abnormalities, resembling congenital AVMs, differ from simple direct fistulous connection between an artery and an adjoining vein, which occurs in normal traumatic injury incurred in any area of the body [2]. Uterine AVMs usually arise in the clinical setting of abortion or pathologic pregnancy followed by dilation and curettage. Indeed, the pathogenesis of uterine AVM is intimately related with pregnancy [15]. It is assumed that such abnormalities arise when venous sinuses become incorporated into the myometrial scar after necrosis of the chorionic villi consequent upon iatrogenic intrauterine procedures or pregnancy-related changes [35].
Abnormal vaginal bleeding is the major presenting symptom, accounting for 1% to 2% of profuse uterine hemorrhage [6]. It has been thought that the vaginal bleeding occurs as the result of the exposure of the AVM from endometrial desquamation or sloughing during menstruation, or iatrogenically during curettage [25]. Bleeding is sometimes severe, and can result in significant anemia or even hypovolemic shock. Rarely, an AVM patient presents with lower abdominal pain, dyspareunia, congestive heart failure or palpable pulsatile mass. Uterine AVM also can be asymptomatic, in most of which cases, it is incidentally detected after hysterectomy [5].
Gray-scale US reveals myometrial inhomogeneity with multiple serpentine, anechoic structures, the diameters of which increase during the Valsalva maneuver. Color Doppler US is the modality of choice for diagnosis of uterine AVM. A typical finding is multi-directional flow that produces a color—mosaic pattern—in the myometrium [3]. Spectral analysis of the vessels within a lesion shows high-velocity arterial flow with a low resistive index [2]. CT or magnetic resonance imaging provides information on lesion location, size, vascularity, and involvement of adjacent organs [8]. Currently, angiography for purely diagnostic purposes is no longer performed [2].
The choice of treatment for uterine AVM accords with the patient's clinical status including severity of vaginal bleeding, comorbidity, and, not least, the patient's desire to preserve fertility [3]. TAE is now generally accepted as the optimal treatment modality for symptomatic uterine AVM [35] and the clinical success rate of TAE is greater than 90% [910]. Badawy et al. [11] reviewed all 25 cases published between 1982 and 1999 and found a success rate after one or two TAE of 96%. Its advantages include excellent success rates, low complication rates, avoidance of surgical risks, and preservation of reproductive function. It has often been cited that, unlike congenital uterine AVMs, acquired AVMs do not have extra-uterine feeding arteries [2]. This fact can facilitate simpler therapy via TAE. Yet, two authors have reported cases of acquired uterine AVM with extra-uterine feeders [68]. Notably, the present case also showed multiple extra-uterine feeders, such as fine branches arising from the anterior branch of the internal iliac artery and the round ligament arteries.
Although radiologic findings on uterine AVMs have been consistently described, diagnosis remains challenging. If accompanied by positive serum β-hCG without recent trauma history, moreover, early diagnosis and determination of management option can be difficult. In the present case, it was presumed that the patient had developed uterine AVM preceded by early pregnancy or retained products of conception, or GTD. TAE was performed for the following reasons: (1) the lesion was, unexpectedly, too extensive for medical treatment alone; (2) the serum hemoglobin level decreased in a short term; and (3) the mildly elevated β-hCG concentration level indicated more toward AVM during early pregnancy or retained products of conception rather than GTD. Even though GTD was a less possible diagnosis, due to lower β-hCG level than the diagnostic criteria, methotrexate was applied to prevent possible complications of GTD. From a clinical perspective, uterine AVM with positive β-hCG of an ambiguous level is a critical situation, because, whereas intractable vaginal bleeding can be treated safely and effectively with TAE, it can prove refractory to conservative treatment or be worsened by curettage.
Certainly, for premenopausal women with unexplained vaginal bleeding, Doppler examination is necessary, and uterine AVM should be included in the differential diagnosis. Associating the patient's clinical history with her imaging findings can facilitate prompt recognition of uterine AVM and ensure optimal treatment. If TAE is planned, it is important to consider the possible presence of extra-uterine feeding arteries.
Figures and Tables
Fig. 1
(A) Transvaginal gray-scale ultrasonography image of complex-echoic mass with numerous irregular, tubular, anechoic structures in uterus. (B) On color Doppler ultrasonography, anechoic structures generated multi-directional and turbulent flow. (C,D) Contrast-enhanced abdominal computed tomography, specifically axial scan (C) in late arterial phase and coronal scan (D) in delayed phase, reveals approximately 8 cm lesion located in anterior myometrium and endometrial cavity. The lesion had intensely enhanced serpentine-like tubular structures, and was accompanied by markedly engorged parametrial (arrows) and ovarian (arrowhead) veins. (E) Initial left uterine artery angiogram showing hypertrophied uterine artery and contrast filling in abnormal vasculature (arrow) within lesion. (F,G) Super-selective angiograms of fine feeder arising from anterior branch of left internal iliac artery (F) and right round ligament artery (G), obtained 5 days after initial transcatheter arterial embolization revealed residual staining of uterine arteriovenous malformation.
References
1. Timmerman D, Van den Bosch T, Peeraer K, Debrouwere E, Van Schoubroeck D, Stockx L, et al. Vascular malformations in the uterus: ultrasonographic diagnosis and conservative management. Eur J Obstet Gynecol Reprod Biol. 2000; 92:171–178.
2. Kwon JH, Kim GS. Obstetric iatrogenic arterial injuries of the uterus: diagnosis with US and treatment with transcatheter arterial embolization. Radiographics. 2002; 22:35–46.
3. O'Brien P, Neyastani A, Buckley AR, Chang SD, Legiehn GM. Uterine arteriovenous malformations: from diagnosis to treatment. J Ultrasound Med. 2006; 25:1387–1392.
4. Grivell RM, Reid KM, Mellor A. Uterine arteriovenous malformations: a review of the current literature. Obstet Gynecol Surv. 2005; 60:761–767.
5. Kelly SM, Belli AM, Campbell S. Arteriovenous malformation of the uterus associated with secondary postpartum hemorrhage. Ultrasound Obstet Gynecol. 2003; 21:602–605.
6. Kim T, Shin JH, Kim J, Yoon HK, Ko GY, Gwon DI, et al. Management of bleeding uterine arteriovenous malformation with bilateral uterine artery embolization. Yonsei Med J. 2014; 55:367–373.
7. Vogelzang RL, Nemcek AA Jr, Skrtic Z, Gorrell J, Lurain JR. Uterine arteriovenous malformations: primary treatment with therapeutic embolization. J Vasc Interv Radiol. 1991; 2:517–522.
8. Bagga R, Verma P, Aggarwal N, Suri V, Bapuraj JR, Kalra N. Failed angiographic embolization in uterine arteriovenous malformation: a case report and review of the literature. Medscape J Med. 2008; 10:12.
9. Maleux G, Timmerman D, Heye S, Wilms G. Acquired uterine vascular malformations: radiological and clinical outcome after transcatheter embolotherapy. Eur Radiol. 2006; 16:299–306.
10. Ghai S, Rajan DK, Asch MR, Muradali D, Simons ME, TerBrugge KG. Efficacy of embolization in traumatic uterine vascular malformations. J Vasc Interv Radiol. 2003; 14:1401–1408.
11. Badawy SZ, Etman A, Singh M, Murphy K, Mayelli T, Philadelphia M. Uterine artery embolization: the role in obstetrics and gynecology. Clin Imaging. 2001; 25:288–295.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download