Abstract
Twin anemia-polycythemia sequence (TAPS) is characterized by a wide discrepancy of hemoglobin between two monochorionic fetuses without sign of twin oligo-polyhydramnios sequence. A primiparous woman with monochorionic diamniotic twin transferred for preterm labor. Ultrasonographic evaluation at 32+3 weeks of gestation revealed increased middle cerebral artery-peak systolic velocity (77.4 cm/sec, 1.69 multiples of median) in donor and decreased in recipient twin (36.4 cm/sec, 0.79 multiples of median), the twin was diagnosed with TAPS. Repeated cesarean section was performed at 32+5 weeks of gestation following preeclampsia and preterm labor. After delivery, TAPS was confirmed through neonatal hematologic examination. There were no signs of acute hemorrhagic shock or brain injury. Placental evaluation via dye infusion and barium angiogram revealed one arterioarterial anastomoses with six arteriovenous anastomoses of placenta. We report a prenatally diagnosed case of spontaneous TAPS with arterioarterial and arteriovenous anastomoses and suggest careful monitoring of monochorionic twin and opinion on placenta vascular architecture.
A large number of monochorionic twin are associated with severe complications through their intertwin vascular anastomoses and unequal placenta sharing ; including growth disturbance and twin to twin transfusion syndrome (TTTS) [1]. Twin anemia-polycythemia sequence (TAPS), unusual form of fetofetal transfusion is first described in 2007 by Lopriore et al. [2]. TAPS is characterized by existence of anemia (with increased reticulocyte count) in the donor and polycythemia in the recipient via few and thin vascular anastomoses of placenta. TAPS can be diagnosed antenatally based on middle cerebral artery (MCA) Doppler ultrasound abnormalities, showing an increased peak systolic velocity (PSV) in the donor and a decreased PSV in the recipient twin [13]. TAPS can developed spontaneously or iatrogenically after fetoscopic laser surgery for TTTS [45].
Clinical course of TAPS vary from mild hemoglobin difference to perinatal demise [6]. The pathogenesis and exact management of TAPS is not known clearly and many attempt was made to identify it. Several studies was reported, detailed information about TAPS is changing gradually. Here, we describe a prenatally diagnosed spontaneous case of TAPS with suspicious arterioarterial (AA) anastomoses of placenta.
A 34-year-old woman gravid 4 para 1was referred to our hospital at 31+2 weeks of gestation for preterm labor and short cervical length reached 1.4 cm. She had previous history of cesarean section. She was pregnant with twin via in vitro fertilization, her prenatal course was uneventful. Intravenous tocolytics and single course of dexamethasone were administered.
Ultrasonographic examination revealed monochorionic diamniotic twin and the placenta was located anterolaterally. The estimated body weight of twin A was 1,778 g (vertex presentation), the twin B was 2131 g (breech presentation). Single deepest pocket (SDP) of twin A was 5 cm, SDP of twin B was 8 cm. There were no structural anomalies in both fetuses on ultrasonographic examination. Due to intertwin weight discrepancy and increased SDP in larger twin, a detailed Doppler examination was done. Doppler examination of the MCA-PSV showed a mild increase (51.3 cm/sec, 1.17 multiples of median [MoM]) in twin A (the donor) and decrease (28.3 cm/sec, 0.64 MoM) in twin B (the recipient). At 32+3 weeks of gestation MCA-PSV Doppler showed further increase in donor (77.4 cm/sec, 1.69 MoM) and still remains low in recipient (36.4 cm/sec, 0.79 MoM), indicative of TAPS. The echogenecity of each placenta territory was clearly divided. Placenta of donor seemed hyperechoic and thicker compared to recipient (Fig. 1). SDP of donor was 4 cm, recipient was 8 cm. Estimated body weight of donor was 1,862 g, recipient was 2,183 g. Tei index of donor was 0.50, recipient was 0.34. There were no signs of hydrops and tricuspid regurgitation in both fetuses.
Repeated cesarean section was performed at 32+5 weeks of gestation following new-onset preeclampsia and redeveloped labor. At delivery, donor was a 2,070-g male infant with Apgar scores of 5 and 8 at 1 and 5 minutes, while recipient was a 2,450-g male with Apgar scores of 5 and 8 (growth differences 16%). The hemoglobin concentration of donor was 11.2 g/dL with reticulocyte counts of 9.28%, while it was 21.3 g/dL with reticulocyte counts of 4.78% in recipient (reticulocyte count ratio 3.78), satisfying the postnatal criteria for TAPS stage 1. After delivery, the donor required a transfusion of 20 mL packed red blood cell (10 mL/kg), the recipient required phlebotomy without exchange transfusion. There were no signs of acute hemorrhagic shock or brain injury.
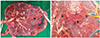
Macroscopic placental examination showed monochorionic diamniotic placenta with a marginal insertion of the umbilical cord of donor and velamentous insertion of the recipient cord. The donor placenta was pale, recipient placenta was reddish congested. Colored agents were added to barium sulphate for achieving accurate visualization of peripheral branches. The arteries and veins of each placenta were injected successively with a 10 mL syringe. After injection, the cords were clamped again to maintain filling of the vessel. Dye injection study revealed seven minuscule anastomoses; six arteriovenous (AV) anastomoses and one AA anastomoses from donor to recipient (Fig. 2). Colored dye did not pass throughout the three AV anastomoses and passed hardly with squeezing and multiple forced injection in the three AV anastomoses. In AA anastomoses colored dye passed easily without resistance, suggestive to in-utero patency. Therefore we suggest that the AA anastomoses caused TAPS development in spite of presence of AV anastomoses. Single-shot digital x-ray barium angiography revealed wider placenta territory of donor twin (donor to recipient ratio, 65:35).
Antenatal and postnatal diagnostic criteria of TAPS are based on staging criteria of Lopriore et al. [2]. TAPS is diagnosed when increased MCA-PSV of >1.5 MoM in one fetus and decreased PSV of <1.0 MoM in the other twin in antenatal Doppler examination without twin oligo-polyhydramios sequence (TOPS). Postnatal TAPS stage is decided by intertwin hemoglobin difference at birth.
We present a prenatally diagnosed spontaneous TAPS suggesting caused by AA anastomoses. AA anastomoses are bidirectional vascular anastomoses, found in uncomplicated monochorionic pregnancy more commonly. In early days, it is known that AA anastomoses of placenta do not cause TAPS and thought to play a protective role against development of TAPS. However, in 2010 van Meir et al. [7] reported a case of TAPS with suspicious AA anastomoses and suggested that the presence of AA anastomoses does not always prevent TAPS. Soon after, Suzuki [8] reported a case of TAPS with only 2 superficial AA anastomoses and suggest that comparable shunt flow through the AA anastomoses might have provoked onset of TAPS. In our case infused colored dye passed through the AA anastomoses easily without resistance, suggestive to in-utero patency, considering that the AA anastomoses may have carried a function in the TAPS development. Colored dye did not pass throughout the three AV anastomoses and passed with multiple forced injection in remnant three AV anastomoses.
Placenta injection study is crucial to understand placenta vascular anastomoses in TAPS, so Lopriore et al. [2] suggested placental findings as the third postnatal criteria of TAPS. There are differences between macroscopic vascular anastomoses at placenta surface and real patent vascular anastomoses via dye injection study. TAPS is specified by slow transfusion between two fetuses in minuscule vessel (diameter <1 mm), so we suggest the involved vascular anastomoses have high possibility to maintain patency immediate after delivery. Practically, lots of study showed vascular anastomoses via dye injection in TAPS.
We recovered other clinical findings during antenatal examination. Prenatal ultrasonographic evaluation revealed differences of placenta echogenecity and thickness as well as MCA-PSV difference. Though there was no TOPS, amniotic fluid discordance was also observed. SDP of recipient twin was increased to 8 cm. Fetal measurement of Tei index revealed that myocardial function of donor twin starts declined until other signs showed up.
Known prevalence rate of TAPS may be underestimated due to underdiagnosis. Measuring MCA-PSV is the only known prenatal diagnostic tool of TAPS. No other ultrasonographic differences can divide monochorionic twin with or without TAPS clearly up to date. However, co-evaluation of placenta appearance and heart function will be helpful as in our case. Vani et al also reported 2 cases of TAPS with discrepancy in placental echogenicity between two fetuses [9].
To determine optimal time to deliver TAPS twins clinicians must consider various factors; gestational age at presentation, severity of TAPS, speed of aggravation and location of placenta. If TAPS is diagnosed in second trimester with accessible placenta, laser coagulation should be tried. With unfavorable placenta location or after failure of laser coagulation, intrauterine transfusion should probably be chosen. While third trimester, serial ultrasonographic examination is suitable for slowly progressed TAPS, delivery is favorable for rapidly progressed TAPS.
Prognosis of TAPS can be appeared from mild hematologic abnormality to irreparable brain injury or perinatal death [610]. Early diagnosis of disease and management such as intrauterine blood transfusion or fetoscopic laser coagulation can change the course of some patients [1112]. Therefore, we suggest essentiality of routine MCA Doppler measurement in monochoronic twins. Late second trimester can be optimal time to start monitoring, at least biweekly serial monitoring is recommended. Especially after laser coagulation for TTTS, in cases of growth discordance and/or AF discordance (even if not TOPS), immediate evaluation of MCA Doppler is recommended [1].
Postnatally, we recommend barium angiogram with colored dye injection while clinicians evaluate placenta. More definite image is obtainable than colored dye infusion solely, division of each placenta territory seemed similar in macroscopic exam is easier. Large scale of study will be helpful to understand placenta vasculature of anastomoses.
Centers treating TTTS by selective fetoscopic laser coagulation is increasing in Korea, therefore incidence of iatrogenic TAPS is expected to increase. Such cases need more careful monitoring and follow-up. Optimal prenatal management in TAPS is under investigation. Several attempt to improve the perinatal outcome was made, such as intrauterine blood transfusion, selective feticide and placenta separation via fetoscopic laser coagulation [613]. Fetoscopic laser coagulation is the only fundamental form of therapy and Slaghekke et al. [11] and Ishii et al. [14] showed better perinatal outcome after laser coagulation especially in spontaneous TAPS in their retrospective cohort study. However, laser coagulation of TAPS is more difficult than that of TTTS. TAPS is diagnosed relatively later than TTTS and absence of stuck twin phenomenon in TAPS also restrict complete therapy due to poor vision. Therefore, large scale of randomized controlled trials is required of establish appropriate management strategy of TAPS.
Figures and Tables
Fig. 1
The placenta of the donor twin seemed hyperechoic and thicker as compared to the placenta of the recipient twin. R, recipient; D, donor.
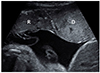
Fig. 2
Colored dye injection study revealed seven minuscule anastomoses; three non-patent arteriovenous anastomoses (open arrows), three hardly passed arteriovenous anastomoses (arrow heads) and one arterioarterial anastomoses (arrow) (A). Details of arterioarterial anastomoses (B). R, recipient; D, donor.
References
1. Slaghekke F, Kist WJ, Oepkes D, Pasman SA, Middeldorp JM, Klumper FJ, et al. Twin anemia-polycythemia sequence: diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther. 2010; 27:181–190.
2. Lopriore E, Middeldorp JM, Oepkes D, Kanhai HH, Walther FJ, Vandenbussche FP. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta. 2007; 28:47–51.
3. Lopriore E, Slaghekke F, Oepkes D, Middeldorp JM, Vandenbussche FP, Walther FJ. Hematological characteristics in neonates with twin anemia-polycythemia sequence (TAPS). Prenat Diagn. 2010; 30:251–255.
4. Groussolles M, Sartor A, Connan L, Vayssiere C. Evolution of middle cerebral artery peak systolic velocity after a successful laser procedure for iatrogenic twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol. 2012; 39:354–356.
5. Herway C, Johnson A, Moise K, Moise KJ Jr. Fetal intraperitoneal transfusion for iatrogenic twin anemia-polycythemia sequence after laser therapy. Ultrasound Obstet Gynecol. 2009; 33:592–594.
6. Lopriore E, Slaghekke F, Kersbergen KJ, de Vries LS, Drogtrop AP, Middeldorp JM, et al. Severe cerebral injury in a recipient with twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol. 2013; 41:702–706.
7. van Meir H, Slaghekke F, Lopriore E, van Wijngaarden WJ. Arterio-arterial anastomoses do not prevent the development of twin anemia-polycythemia sequence. Placenta. 2010; 31:163–165.
8. Suzuki S. Twin anemia-polycythemia sequence with placental arterio-arterial anastomoses. Placenta. 2010; 31:652.
9. Movva VC, Rijhsinghani A. Discrepancy in placental echogenicity: a sign of twin anemia polycythemia sequence. Prenat Diagn. 2014; 34:809–811.
10. Lopriore E, Slaghekke F, Oepkes D, Middeldorp JM, Vandenbussche FP, Walther FJ. Clinical outcome in neonates with twin anemia-polycythemia sequence. Am J Obstet Gynecol. 2010; 203:54.e1–54.e5.
11. Slaghekke F, Favre R, Peeters SH, Middeldorp JM, Weingertner AS, van Zwet EW, et al. Laser surgery as a management option for twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol. 2014; 44:304–310.
12. Slaghekke F, van den Wijngaard JP, Akkermans J, van Gemert MJ, Middeldorp JM, Klumper FJ, et al. Intrauterine transfusion combined with partial exchange transfusion for twin anemia polycythemia sequence: modeling a novel technique. Placenta. 2015; 36:599–602.
13. Assaf SA, Benirschke K, Chmait RH. Spontaneous twin anemia-polycythemia sequence complicated by recipient placental vascular thrombosis and hydrops fetalis. J Matern Fetal Neonatal Med. 2011; 24:549–552.
14. Ishii K, Hayashi S, Mabuchi A, Taguchi T, Yamamoto R, Murata M, et al. Therapy by laser equatorial placental dichorionization for early-onset spontaneous twin anemia-polycythemia sequence. Fetal Diagn Ther. 2014; 35:65–68.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download