Abstract
Objective
The purpose of this case series was to retrospectively examine records of cases with uterine rupture in pregnancies following myomectomy and to describe the clinical features and pregnancy outcomes.
Methods
This study was conducted as a multicenter case series. The patient databases at 7 tertiary hospitals were queried. Records of patients with a diagnosis of uterine rupture in the pregnancy following myomectomy between January 2012 and December 2014 were retrospectively collected. The uterine rupture cases enrolled in this study were defined as follows: through-and-through uterine rupture or tear of the uterine muscle and serosa, occurrence from 24+0 to 41+6 weeks’ gestation, singleton pregnancy, and previous laparoscopic myomectomy (LSM) or laparotomic myomectomy (LTM) status.
Results
Fourteen pregnant women experienced uterine rupture during their pregnancy after LSM or LTM. Preterm delivery of less than 34 weeks’ gestation occurred in 5 cases, while intrauterine fetal death occurred in 3, and 3 cases had fetal distress. Of the 14 uterine rupture cases, none occurred during labor. All mothers survived and had no sequelae, unlike the perinatal outcomes, although they were receiving blood transfusion or treatment for uterine artery embolization because of uterine atony or massive hemorrhage.
Conclusion
In women of childbearing age who are scheduled to undergo LTM or LSM, the potential risk of uterine rupture on subsequent pregnancy should be explained before surgery. Pregnancy in women after myomectomy should be carefully observed, and they should be adequately counseled during this period.
Uterine myomas are clinically observed in 20% to 25% of women of reproductive age [1]. Although uterine myoma symptoms may vary depending on their size and position, they are known to affect pregnancy outcomes, causing subfertility, pregnancy complications, and pregnancy loss [23]. In the management of uterine myoma during childbearing age, many surgical or medical methods have been attempted, but laparotomic myomectomy (LTM) or laparoscopic myomectomy (LSM) remains the most widespread. Reflecting the growing trend of delayed childbearing in Korea, the incidence of pregnant women with uterine myomas or who have undergone surgical treatment for myoma is gradually increasing. Although considerable discussion has been devoted to concerns of uterine rupture based on the invasive myomectomic approach involved, the risk of uterine rupture during pregnancy after myomectomy remains unknown and appears to be as high as that of a previous cesarean section. In particular, myomectomy for intramural type myoma before pregnancy can lead to uterine rupture during labor and sometimes even early in pregnancy [4].
Pregnancy after myomectomy may increase the risk of intrauterine adhesions, miscarriage, preterm birth, abnormal placentation, cesarean section, and uterine rupture [56]. Above all, uterine rupture during pregnancy is a cause of stillbirth, perinatal hypoxic brain damage, cerebral palsy, and intrauterine fetal death (IUFD) of the fetus [7]. A recent single center study reported that uterine rupture after LSM occurred in only 3 of 523 pregnancies (0.6%), miscarriage occurred in 13%, preterm deliveries in 10.4%, and full-term deliveries in 76.7% [8]. A meta-analysis showed that the risk of uterine rupture was 0.4% following LTM and 1.2% in LSM. In addition, neonatal mortality related to uterine rupture occurred in 33%. These studies asserted that myomectomy for women of reproductive age could be relatively safe [9]. However, the incidence of uterine rupture may vary in function of the size, type, and location of the myoma, in addition to the suture technique used for myomectomy, and the interval of subsequent pregnancy and myomectomy [1011]. The World Fibroid Registry has clearly indicated that uterine rupture after LTM is grossly under reported or overlooked [1213], since it is possible that cases of unsuspected uterine rupture without any serious sequelae may not have been reported.
Considering these controversial reports and the potential impact of uterine rupture in the pregnant woman, it is necessary that cases for uterine rupture in a pregnancy following myomectomy be collected from multiple-centers because of their low incidence. The purpose of this case series was to retrospectively examine records of cases with uterine rupture in a pregnancy following myomectomy and to describe the clinical features and pregnancy outcomes.
This study was conducted as a multicenter case series. The patient databases at 7 tertiary care hospitals were queried. Records of patients with a diagnosis of uterine rupture in a pregnancy following myomectomy between January 2012 and December 2014 were retrospectively collected. Clinical characteristics and pregnancy outcomes were obtained from each case. The uterine rupture cases enrolled in this study were defined as follows: through-and-through uterine rupture or tear of the uterine muscle and serosa (silent uterine rupture) [14], occurrence from 24+0 to 41+6 weeks’ gestation, singleton pregnancy, and previous LSM or LTM status. Pregnant women with multiple pregnancies, uterine rupture occurring before 24+0 weeks’ gestation, and treatment for uterine myomas other than LSM or LTM were excluded from the series.
In this study, we identified and reviewed the medical records of 14 pregnant women who had uterine rupture after LSM or LTM. Clinical and surgical characteristics for the uterine rupture cases are shown in Table 1. Preterm delivery at less than 34 weeks’ gestation occurred in 5 cases, IUFD in 3 cases, and fetal distress in 3 cases. Among the 14 cases of uterine rupture reported, none occurred during labor. Unlike perinatal outcomes, all mothers survived and had no sequelae despite receiving blood transfusion or treatment of uterine artery embolization because of uterine atony or massive hemorrhage.
The cases (cases 2, 7, 8, 11, 12, 13, and 14) that did not exhibit any rupture symptoms were not suspected of uterine rupture and had relatively mild adverse pregnancy outcomes (Fig. 1). In these cases, most of the ruptures were relatively small or had intact fetal membranes (silent uterine rupture). The cases (case 1, 3, 4, 5, 6, 9, and 10) with symptoms suspected to be uterine rupture or fetal distress showed poor pregnancy outcomes (Fig. 2). These serious uterine rupture cases resulted in poor perinatal outcomes, such as IUFD and perinatal asphyxia, and had larger rupture size than the others.
A pregnant woman at 27+6 weeks’ gestation, who developed severe epigastric pain and diarrhea, was admitted for conservative management and fetal surveillance monitoring. After 2 days, fetal heart rate was suddenly undetectable by transabdominal ultrasound. She was transferred to a tertiary hospital under the suspicion of IUFD and placental abruption. On admission, there was continuous pain on the uterine fundal area, local and hypertonic uterine contraction with the suspicion of placental abruption. Abdomen computed tomography and ultrasonography were performed. She was diagnosed with moderate hemoperitoneum and uterine rupture with protrusion of the amniotic cavity and placenta (Fig. 2D). Emergent hysterotomy was decided and a uterine defect of 5×5 cm in size was repaired on the fundus.
The patient was initially admitted to a district hospital at 27+6 weeks’ gestation because of severe abdominal pain. Tocolytics were administered because of regular contractions every 4 minutes as detected by cardiotocography. The vital signs of the pregnant women were within normal range and her abdominal pain subsided after using tocolytics. However, 2 hours and 40 minutes later, she underwent an emergency cesarean section due to acute vaginal bleeding. A small-sized uterine rupture on the anterior uterine corpus was detected after the newborn was delivered.
A 32-year-old pregnant woman, who was at 31+4 weeks’ gestation, visited the antenatal care center due to abdominal pain with vaginal bleeding to the degree that it rubbed off on underwear. She was transferred to a tertiary hospital under the suspicion of placental abruption and IUFD. Entering into abdominal cavity, massive blood clots and the fetus enclosed by amniotic membrane on the right upper abdominal cavity were observed. The uterus was longitudinally ruptured from 1/5 of the upper segment to the lower segment on the posterior right side (Fig. 2C). The rupture was largely repaired. The entire procedure was well tolerated by the patient.
The primipara at 33+6 weeks of gestation was admitted to the hospital with continuous, severe right flank pain that had begun 2 days prior. Irregular uterine contraction and variable deceleration every 4 to 5 minutes on cardiotocography were observed. Tocolytics were used to reduce uterine contractions and oxygen was applied, but fetal variability decreased with regular contraction by 2 or 3 minutes. Fetal heart rate fell by 60 beats per minute. A prolonged deceleration for about 5 minutes was observed. Thus, an emergency cesarean section was performed. A 4×4-cm-sized uterine rupture and hemoperitoneum were noted during the procedure. The 1- and 5-minute Apgar score of the neonate was 1 and 4, respectively.
The mother was admitted to an emergency room at 33+6 weeks’ gestation because of severe abdominal pain. On the cardiotocography conducted after admission, contractions at 1- to 2-minute intervals were measured. Although tocolytics were administered for 4 days, uterine contractions persisted at 1- to 3-minute intervals, and a non-reassuring fetal heart rate suddenly developed. The pregnant woman underwent an emergency cesarean section. A rupture of 6 cm was noted on the fundus of the uterus. Although she received transfusion, the mother was discharged without any particular sequelae. The 1- and 5-minute Apgar scores of the neonate were 2 and 5, respectively.
The patient was transferred the emergency room at 34+3 weeks’ gestation from the antenatal care center, experiencing severe epigastric pain with nausea 3 days prior. She complained of abdominal pain on the right lower quadrant area at the time admission. An ultrasound ruled out appendicitis and confirmed that there was no abnormality on the appendix. No fetal abnormal finding was observed either on the nonstress test or on ultrasonography. Subsequently, the fetal heart rate suddenly decreased to 80 bpm for 5 minutes; thus, an emergency cesarean section was ordered. During the procedure, a uterine rupture of 6 cm was observed longitudinally along the posterior right uterine wall, with blood and hematoma of about 1,000 mL (Fig. 2A). The ruptured site was successfully repaired and neither infant nor mother had any subsequent sequelae.
A pregnant woman, at 35+4 weeks of gestation, was admitted to the hospital for elective cesarean section due to preterm labor with a uterine rupture. She had undergone myomectomy several years previously. When the peritoneum was opened, her uterus seemed to be near full-term size and a horizontal rupture of the uterine wall was noted on the right fundus of about 8 cm in length, but the fetal membranes were intact. The Apgar score of the neonate was 7 at 1 minute and 9 at 5 minutes. She and the infant had no specific problems following birth.
The patient was admitted to hospital at 35+6 weeks of gestation with continuous, severe left flank pain. There was labor of 5-minute intervals on the nonstress test. Her vital signs were normal. On opening of the peritoneum, hemoperitoneum was found in the abdominal cavity. After the delivering a male infant weighing 2,510 g, the rupture site was no longer visible. The uterus was exposed to the outside of abdominal cavity, in order to repair the uterine wall. The rupture site was found on the left posterior wall appearing as a hole (Fig. 1A, B). The rupture was successfully repaired. The patient tolerated the entire procedure and was sent to the recovery room with good vital signs.
A 39-year-old pregnant woman at 36+3 weeks’ gestation suffered abdominal pain with regular labor contractions at 3- to 5-minute intervals and cervical dilatation of 2 cm. Emergent cesarean section was decided, and a longitudinal rupture of the posterior lateral wall of the uterus of about 10 cm was found during the procedure (Fig. 2B). The ruptured uterus was successfully repaired, but postpartum bleeding due to uterine atony persisted. Following the operation, uterine artery embolization was performed because of uterine atony bleeding.
The patient, at 37+4 weeks of gestation, visited the hospital to receive antenatal care, and complained of low abdominal pain with unusual vaginal spotting 2 days prior. She was quickly discharged without any specific findings on examination. The pregnant woman returned to hospital because of worsening abdominal pain on the same day of discharge. At the time of admission, because of the patient’s anemic facial features and fetal bradycardia, she was transferred to a tertiary hospital under the diagnosis of placental abruption. The initial vital signs showed blood pressure was 80/50 mmHg, the heart rate was 102 beats per minute, and the respiratory rate was 18 per minute. Initial hemoglobin concentration was 6.3 g/dL. On ultrasonography, the fetal heart rate was not detected. During surgery, the fetal membranes were ruptured and an approximately 10-cm uterine rupture was found at the fundal area of the uterus. The fetus was located outside the uterus. In this case, the patient was not aware of the mode of delivery after myomectomy, thus she was unable to report her operation history to the obstetrician in any specific detail.
A 39-year-old pregnant woman at 37+6 weeks’ gestation was admitted to hospital for elective cesarean section. She had undergone LSM for a 3-cm-sized uterine myoma several years prior. During elective surgery for delivery, it was confirmed that the serosa of the uterus was swelled at the same site of the myomectomy on the right anterior fundus. The defect was successfully repaired without any subsequent complications.
A woman received elective cesarean section at 38+1 weeks’ gestation. She had no other obstetrical problems except having received a previous myomectomy. Elective cesarean section was performed. During the operation, an approximately 3-cm serosal swelling without rupture was found, which was covered by fetal membranes only (Fig. 1C, D). After the fetus was delivered, the defect was successfully repaired.
A pregnant woman was hospitalized at 38+3 weeks gestation for elective cesarean section. There were no specific findings prior to surgery. She was subjected to LTM 12 months prior. On entering the abdominal cavity to deliver the fetus, an approximately 10×10-cm-sized defect on the anterior wall of the uterus was observed. Fortunately, there was no active bleeding and the fetal membranes were intact (silent rupture) (Fig. 1E, F). The fetus was successfully delivered through the defective site, and the defect on the uterine wall was successfully repaired.
A 43-year-old pregnant woman at 38+4 weeks’ gestation, who had undergone myomectomy before pregnancy was admitted to hospital for elective cesarean section. On entering the abdominal cavity for cesarean section, the maternal side of the placenta was observed partially exposed to the abdominal cavity due to uterine wall dehiscence and the uterine defect consisted of adnexal and peritoneal adhesions. Both infant and mother had no complications following delivery.
A uterine rupture can occur with a variety of clinical manifestations. The impact on the mother and her fetus is also very diverse. The risk of uterine rupture by LSM was reported to be about 0.6% [8] and was similar to that of LTM [9]. In our 14 case series, the number of uterine rupture cases after LSM were 8 (8/14, 57%). Regardless of the operating method applied as in previous reports, the risk of uterine rupture seemed to be similar. To date, various hypotheses regarding the risk of uterine rupture in pregnancy after myomectomy have been suggested in many studies. In addition, there has been controversy regarding the relationship between uterine rupture in the pregnancy following myomectomy and the myoma type, location, cavity involvement, suture layer, time interval, gestational age, operation type, and uterine contraction. A recent meta-analysis suggested that the risk of uterine rupture after myomectomy was 0.75%, and the rupture risk was constant regardless of the surgical technique or myoma size [9]. A notable finding was that in only one case the rupture occurred during labor, while in the remaining (28 cases) the rupture occurred before the onset of labor. Most of the ruptures (80%) occurred during the preterm period (between 28 and 36 weeks’ gestation). A systemic review showed that the overall incidence of uterine rupture from 11 studies was 0.93% (0.45% to 1.92%) (n=7/756) and was 0.47% (0.13% to 1.70%) (n=2/426) in women undergoing labor after myomectomy. Of the 7 uterine ruptures reported, 5 (71%) occurred within 36 weeks of gestation [15]. In this case series, the gestational age of the uterine ruptures varied between 27+6 and 38+4 weeks’ gestation, LSM had been previously performed in 8 cases (8/14, 57%), the time interval between the uterine rupture and myomectomy was 12 to 84 months, and most of the uterine ruptures were found in absence of uterine contraction.
Cases 7, 13, and 14 were referred to as silent uterine ruptures. These cases shared the fact that there were no obvious symptoms during pregnancy, uterine rupture was detected at the time of elective surgery, the ruptures were found in the absence of labor in the period near term, and the maternal and fetal outcomes were all favorable. Cases 2, 8, 11, and 12 had a small-sized uterine rupture. The rupture size was 2 or 3 cm in diameter and took the form of a hole. These cases also had good fetal and maternal pregnancy outcomes. However, in cases 1, 3, 4, 5, 6, 9, and 10, the size of the uterine rupture was relatively greater than 5 cm. Three fetuses died (3/14, 21%) before cesarean section, and 3 fetuses had adverse pregnancy outcomes including perinatal asphyxia and a poor Apgar score. In particular, these were very serious IUFD occurring in cases 1, 3 and 10. Most pregnant women with a large-sized uterine rupture experienced massive post-partum bleeding due to uterine atony or rupture and received transfusions.
Although surgical information for the uterine rupture in the series were amply collected from the 7 tertiary hospitals participating in the study, information regarding the operative history including size, type, and location of the uterine myoma, and suture methods used at the uterus incision site was limited because of scant information available from the previous surgery for uterine myomas. Cases 1 and 3, in particular were severe uterine rupture cases, and the outcome of their pregnancies were fetal death. The 2 patients knew very few details regarding their previous myomectomies. Despite the various clinical findings from these 14 cases, the risk factors affecting pregnancy outcomes may not have been properly analyzed.
Compared to the risk of a prior cesarean section and myomectomy, the overall risk for uterine rupture was reported to be 0.32% in pregnant women with a previous cesarean section [16], and from approximately 0.6% to 0.8% in women with myomectomy [89]. Moreover, it is possible that most previous studies regarding uterine ruptures may not have included cases with silent or small-sized uterine rupture in the analyses. Since the mother and neonate did not exhibit any sequelae after delivery, a silent uterine rupture might not have been referred to the mother or may have been ignored altogether. These uterine ruptures without adverse pregnancy outcomes are not to be considered safe, but only lucky. Because of the potential of these missing ruptures not being reported, the overall risk of uterine rupture may have been underestimated in this study. The actual risk of uterine rupture in pregnancy after myomectomy might actually be higher than that of previous reports. Furthermore, it is possible that the uterine rupture having an abrupt onset is mistaken for a preterm labor or placental abruption. This misimpression may cause a delay in timing of adequate treatment. Thus, pregnant women with previous myomectomy should be counseled more carefully for the risk of the uterine rupture.
Given the potential impact of uterine rupture to mothers and their fetuses, it is essential that obstetricians perform careful follow-ups for women during a pregnancy after myomectomy. Moreover, they should provide adequate counseling and explain the potential risk of uterine ruptures occurring during a future pregnancy after myomectomy to women and their families before the myomectomy procedure. In conclusion, it is possible that there may be different types of uterine rupture, and, analogously, there may be a variety of clinical characteristics. However, uterine rupture can lead to fatal consequences for both the fetuses and the pregnant mother. Thus, uterine rupture may ultimately not be such an extremely rare complication. Therefore, if a woman of childbearing age is scheduled to undergo LTM or LSM, the risk of uterine rupture that may occur during subsequent pregnancy should be clearly explained before surgery. All pregnant women after myomectomy should be carefully observed and counseled during pregnancy period.
Figures and Tables
Fig. 1
Cases unsuspected of uterine rupture after myomectomy. Uterine rupture of the ‘hole’ type can be seen at the posterior uterine wall after placental delivery (case 8; A, B), and the myometrium defect (black arrow) is enclosed with the serosal membrane at the fundus of uterus (case 12; C, D). After peritoneal incision, the serosal and myometrial defects of uterus were noted incidentally and the intact fetal membranes remained and protruded forward (case 13; E, F).
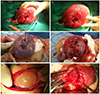
Fig. 2
Serious uterine rupture cases after myomectomy with adverse pregnancy outcomes. (A) An approximately 6-cm-sized longitudinal tear can be seen at the right posterior uterine wall, with massive bleeding (case 6). (B) Longitudinal uterine rupture can be noted at right posterior lateral wall (case 9). (C) The uterus is torn longitudinally and ruptured throughout the right posterior wall of the uterus (case 3). (D) Before emergency cesarean section, the fetus was stillborn. Uterine rupture with protrusion of amniotic cavity and placenta, massive hemoperitoneum, and the uterine wall defect (white arrow) are found on abdominal computerized tomography (case 1).

References
1. Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981; 36:433–445.
2. Carlson KJ, Miller BA, Fowler FJ Jr. The maine women’s health study: I. outcomes of hysterectomy. Obstet Gynecol. 1994; 83:556–565.
3. Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998; 70:687–691.
4. Brady PC, Stanic AK, Styer AK. Uterine fibroids and subfertility: an update on the role of myomectomy. Curr Opin Obstet Gynecol. 2013; 25:255–259.
5. Parazzini F, Tozzi L, Bianchi S. Pregnancy outcome and uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016; 34:74–84.
6. Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008; 198:357–366.
7. Sinha M, Gupta R, Gupta P, Rani R, Kaur R, Singh R. Uterine rupture: a seven year review at a tertiary care hospital in New Delhi, India. Indian J Community Med. 2016; 41:45–49.
8. Koo YJ, Lee JK, Lee YK, Kwak DW, Lee IH, Lim KT, et al. Pregnancy outcomes and risk factors for uterine rupture after laparoscopic myomectomy: a single-center experience and literature review. J Minim Invasive Gynecol. 2015; 22:1022–1028.
9. Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systemic review of the literature and meta-analysis. Gynecol Surg. 2014; 11:197–206.
10. Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010; 17:551–554.
11. Hackethal A, Westermann A, Tchartchian G, Oehmke F, Tinneberg HR, Muenstedt K, et al. Laparoscopic myomectomy in patients with uterine myomas associated with infertility. Minim Invasive Ther Allied Technol. 2011; 20:338–345.
12. Trivedi P, Abreo M. Predisposing factors for fibroids and outcome of laparoscopic myomectomy in infertility. J Gynecol Endosc Surg. 2009; 1:47–56.
13. Bernardi TS, Radosa MP, Weisheit A, Diebolder H, Schneider U, Schleussner E, et al. Laparoscopic myomectomy: a 6-year follow-up single-center cohort analysis of fertility and obstetric outcome measures. Arch Gynecol Obstet. 2014; 290:87–91.
14. Iemura A, Kondoh E, Kawasaki K, Fujita K, Ueda A, Mogami H, et al. Expectant management of a herniated amniotic sac presenting as silent uterine rupture: a case report and literature review. J Matern Fetal Neonatal Med. 2015; 28:106–112.
15. Gambacorti-Passerini Z, Gimovsky AC, Locatelli A, Berghella V. Trial of labor after myomectomy and uterine rupture: a systematic review. Acta Obstet Gynecol Scand. 2016; 95:724–734.
16. Spong CY, Landon MB, Gilbert S, Rouse DJ, Leveno KJ, Varner MW, et al. Risk of uterine rupture and adverse perinatal outcome at term after cesarean delivery. Obstet Gynecol. 2007; 110:801–807.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download