Abstract
Umbilical cord ulceration is a rare condition presenting with sudden fetal bradycardia due to fetal hemorrhage and in most cases leading to intrauterine death. A strong association with intestinal atresia has been reported. Most cases present after 30 weeks of gestation, with preterm labor or rupture of membranes followed by sudden fetal bradycardia. We report two such cases of umbilical cord ulceration and review the available literature. One of the cases interestingly presented at 26 weeks, much earlier than what is reported in the world literature. In view of high perinatal mortality and morbidity, awareness of this condition is mandatory for timely and appropriate management to improve the fetal outcome.
Umbilical cord ulceration (UCU) is an under-reported, but important cause of massive fetal hemorrhage which was first reported by Bendon et al. in 1991 [1]. A review of literature revealed only 55 such cases, in which clinically significant intrauterine hemorrhage was produced from UCU occurring in association with fetal intestinal atresia [2]. We report two cases of UCU with the aim to highlight the importance of suspecting and diagnosing these cases during antenatal checkups. This will ensure the segregation of such high risks cases requiring rigorous monitoring and immediate delivery at the first sign of fetal hypoxia. Such a practice may help in improving fetal outcome.
A 28-year-old woman, gravid-three, with previous two uncomplicated term pregnancies came to hospital at 26 weeks of gestation with complaints of pain in abdomen and bleeding per vagina. She had an uneventful antenatal course at a private clinic until admission at our hospital. Abdominal examination revealed a uterine size increased for the gestational age. Fetal heart rate was 110 to 120 beats/min. Vaginal speculum examination showed a closed cervical os with evidence of fresh blood. An emergency ultrasonography revealed a single live intrauterine fetus. "Double bubble" sign, suggestive of duodenal atresia along with polyhydramnios were also present. Placenta was inserted at normal position. Steroids and tocolytics were administered and continuous fetal heart rate monitoring was done. However, few hours later, the fetus suddenly developed persistent bradycardia (60 beats/min) and the patient delivered a female still born baby by emergency lower segment cesarean section (LSCS), weighing approximately 1,200 g. Heavily blood stained liquor was drained. The baby was grossly pale at birth. No postpartum hemorrhage was noted and mother was stable. The placenta was sent for histopathological examination.
The placenta weighed 300 g and measured 14×9×4 cm in size. There was no evidence of retroplacental clot or infarction. The cord was centrally inserted, measured 16 cm in length and had three lumina. A linear ulcer measuring 2 mm in length with an overlying adherent blood clot (Fig. 1A) was identified at a distance of 10 cm from the point of placental insertion. Microscopic examination revealed marked aneurysmal dilatation of one of the vessels (Fig. 1B) with focal rupture of its muscular wall (Fig. 1C) and evidence of degeneration as well as hemorrhage into the Wharton's jelly (Fig. 1D). Additional findings included chorioamnionitis and extensive areas of placental calcification. Pigment containing macrophages, though looked for, were not identified. Consent for fetal autopsy was declined.
A 24-year-old woman, second gravid, with prior history of LSCS done for contracted pelvis, was referred from a private hospital at 38 weeks of gestation with complaints of non perception of fetal movements. Her antenatal history card did not mention any prior significant event. On examination, the uterine size corresponded to 40 weeks of gestation. Cervical os was closed; however there was evidence of watery discharge per vaginum indicative of premature rupture of membranes. Emergency ultrasonography revealed absence of cardiac activity in the fetus along with polyhydramnios with a single pocket measuring 8 cm. Multiple cystic structures were noted in the fetal abdomen suggestive of intestinal atresia. Placenta was normally inserted. A markedly pale baby was delivered by emergency LSCS. There were no retroplacental clots. Estimated blood loss of mother was average. Regretfully, autopsy of baby could not be performed due to social beliefs.
Placenta measured 13×2×2 cm and weighed 510 g. The attached cord was 12 cm long and cut section showed three lumina. Microscopic sections from the cord revealed marked aneurysmal dilatation and thinning of muscular wall of two of the umbilical vessels along with focal rupture leading to hemorrhage from one of these into perivascular spaces and Wharton's jelly (Fig. 2A). Although, grossly there was no surface ulceration, microscopic evidence of focal surface hemorrhage was noted. This indicated the possibility of a small breach in the amniotic epithelium (Fig. 2B). Membranes revealed mild chorioamnionitis. No pigment laden macrophages were identified. The placenta was histologically unremarkable.
UCU and fetal hemorrhage in relation to congenital intestinal atresia were first reported by Bendon et al. in 1991 [1]. Since then a total of 55 such cases have been reported in literature, the bulk of which has been from Japan [2]. The site of intestinal obstruction has either been the duodenum or the jejunum, i.e., distal to the ampulla of Vater with no association with esophageal atresia reported till date [3].
The exact pathogenesis is unclear. However, three major possible mechanisms had been proposed by Bendon et al. [1]. These include vascular theory, reflux theory, and epithelial abnormality [1]. It has been suggested that vascular hyper-reactivity and secondary ischemia may lead to simultaneous occurrence of both UCU as well as intestinal atresia. The second theory implicates that reflux of gastric or intestinal contents (digestive enzymes like pepsin, trypsin, bile and bilirubin) owing to intestinal obstruction can lead to UCU. This theory is supported by the evidence of increased bile acid concentrations (hemosiderin negative green brown pigment) in amniotic fluid of affected fetuses and presence of such pigment laden macrophages in the ulcer beds in these cases. Absence of UCU in infants with atresia proximal to ampulla of Vater also validates this hypothesis [4]. UCU in such cases should be multifocal with random distribution rather than being linear along the length of the vasculature. Yet another theory proposes primary epithelial abnormality similar to the association of epidermolysis bullosa with intestinal atresia as the cause of UCU [5]. Altshuler et al. [6] postulated meconium to be a possible irritant in their study on 10 cases of UCU. Other agents including cigarette smoking, persistent omphalomesenteric duct, complete absence of Wharton's jelly, injury by fetal nail have also been implicated [7]. Sadly, none of the above discussed hypotheses is perfect enough to be valid in all the cases.
Like the etiology, the exact incidence of this association is also not known. The entity is grossly under-reported due to lack of awareness. In addition, very small ulcers may be missed macroscopically [4]. In our patients, the second case failed to show any surface ulceration. Also less severe cases, lacking significant fetal hemorrhage are usually not identified, and the actual incidence may be much higher if umbilical cords and placentae of all cases of intestinal atresia are histopathologically evaluated. Ohyama et al. [8] in their study on placentae and umbilical cords from 29 cases of congenital upper intestinal atresia had found only six (14%) to show UCU, all of which had led to life-threatening fetal hemorrhage [6]. Ichinose et al. [9] analyzed umbilical cords of 20 patients of duodenal/jejunal atresia and compared the results with 28 control cases. The authors graded the degree of UCU as: grade 1, desquamation of epithelium only; grade 2, detachment of basal lamina; grade 3, thinning of the Wharton's jelly with widespread grade 2 change; and grade 4, exposed umbilical artery or vein. Six of the 28 (21%) controls showed grade 1 ulceration. All but one of the 20 cases (95%) was found to have different degrees of UCU (three grade 1, seven grade 2, seven grade 3, and two grade 4) [9]. This implies that most of the cases, especially the lower grade lesions, which did not lead to clinically significant hemorrhage, go undiagnosed. Using the criteria laid down by Ichinose et al. [9], we found the our two cases to have grade 4 UCU.
Symptoms usually begin with the onset of premature labor or rupture of membranes. An increase in intra-uterine pressure with the onset of labor causes rupture of the umbilical vessel into the amniotic cavity triggering massive fetal hemorrhage and subsequent bradycardia. Though very few cases of UCU have been picked up antenatally by ultrasonography [41011], the low number points rather to the lack of awareness of such complication than to the difficulty in diagnosing it. Interestingly, UCU has been reported to be located near the fetal side in most of the reported cases and hence, a meticulous ultrasonographic evaluation of the umbilical cord structure especially at the fetal end in high-risk cases is advisable [7]. Measurement of bile acid concentration in amniotic fluid can further help in segregation of high risk cases [4].
Although one of our case was exceptional in presenting at 26 weeks, almost all cases reported till date in the literature presented after 30 weeks of gestation [5]. This is important as a gestational age of 30 weeks can be taken as an alert line beyond which regular monitoring may be required. At its simplest, continuous fetal heart monitoring at the onset of premature labor or premature rupture of membrane in a case of polyhydramnios with fetal intestinal atresia has been found to improve the outcome [4].
In conclusion, UCU is associated with intestinal atresia and sudden massive fetal hemorrhage heralding poor fetal outcome unless prompt intervention is initiated with immediate delivery at the first signs of fetal hemorrhage/ hypoxia.
Figures and Tables
Fig. 1
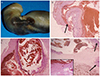
Case 1. (A) Gross specimen showing surface ulceration of the umbilical cord with overlying hematoma. (B) Microscopic counterpart showing breach in the amniotic epithelium (H&E, ×100) (arrow). (C) Section showing umbilical vessel dilatation with focal rupture and hemorrhage into adjoining perivascular space (H&E, ×100). (D) Section at another level of the cord showing two uninvolved vessels (arrows). There is evidence of aneurysmal dilatation of the third vessel with marked loss of its muscular wall (H&E, ×100). Adjoining Wharton's jelly is showing hemorrhage and degeneration (inset; H&E, ×200) is noted.
References
1. Bendon RW, Tyson RW, Baldwin VJ, Cashner KA, Mimouni F, Miodovnik M. Umbilical cord ulceration and intestinal atresia: a new association? Am J Obstet Gynecol. 1991; 164:582–586.
2. Ohyama M, Itani Y, Ishikawa H, Tanaka Y. Is umbilical cord ulcer associated with congenital upper intestinal atresia so rare? Japanese case series and review of the literature. Fetal Diagn Ther. 2010; 28:236–237.
3. Yamanaka M, Ohyama M, Koresawa M, Kawataki M, Ohsaki I, Tanaka Y. Umbilical cord ulceration and intestinal atresia. Eur J Obstet Gynecol Reprod Biol. 1996; 70:209–212.
4. Shimizu S, Kawagishi R, Arimoto-Ishida E, Wada K, Shimoya K, Murata Y. Fetal hemorrhage associated with congenital intestinal atresia. J Obstet Gynaecol Res. 2003; 29:312–316.
5. Chan SS, Lau AP, To KF, Leung TY, Lau TK, Leung TN. Umbilical cord ulceration causing foetal haemorrhage and stillbirth. Hong Kong Med J. 2008; 14:148–151.
6. Altshuler G, Arizawa M, Molnar-Nadasdy G. Meconium-induced umbilical cord vascular necrosis and ulceration: a potential link between the placenta and poor pregnancy outcome. Obstet Gynecol. 1992; 79(5 Pt 1):760–766.
7. Anami A, Morokuma S, Tsukimori K, Kondo H, Nozaki M, Sueishi K, et al. Sudden fetal death associated with both duodenal atresia and umbilical cord ulcer: a case report and review. Am J Perinatol. 2006; 23:183–188.
8. Ohyama M, Itani Y, Yamanaka M, Imaizumi K, Nishi T, Ijiri R, et al. Umbilical cord ulcer: a serious in utero complication of intestinal atresia. Placenta. 2000; 21:432–435.
9. Ichinose M, Takemura T, Andoh K, Sugimoto M. Pathological analysis of umbilical cord ulceration associated with fetal duodenal and jejunal atresia. Placenta. 2010; 31:1015–1018.
10. Miyake H, Yamamoto A, Yamada T, Okazaki K, Morita K, Kondo M, et al. Umbilical cord ulceration after prenatal diagnosis of duodenal atresia with interstitial deletion of chromosome 13q: a case report. Fetal Diagn Ther. 2008; 24:115–118.
11. Nijagal A, Rand L, Goldstein R, Poder L, Miniati D. Intrauterine umbilical cord hemorrhage with associated jejunal atresia captured by real-time ultrasound. Am J Obstet Gynecol. 2009; 200:e5–e6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download