Abstract
Objective
This study was designed to review the screening performance of combined test at the Ewha Womans University Mokdong hospital.
Methods
All women admitted for routine antenatal care between January 1st 2008 and December 31st 2012 with a known pregnancy outcome were included in this study, totaling 1,156 women with singleton pregnancies presenting at 10 to 13 weeks of gestation. Women were offered screening using a combination of maternal serum pregnancy-associated plasma protein-A, free β-human chorionic gonadotropin and fetal nuchal translucency thickness. Those with an estimated risk of ≥1 in 250 of carrying a fetus with trisomy 21 or ≥1 in 300 risk of trisomy 18 were offered genetic counseling with the option of an invasive diagnostic test.
Results
The median of gestational age was 11+3 weeks, the median of crown-rump length was 47.1 mm, and the median age of the women was 31 years. The detection rate was 80% for trisomy 21 (4 of 5) and 100% for trisomy 13 and 18 (all 2). The false-positive rate was 7.73% for trisomy 21 and 1.21% for trisomy 18.
Conclusion
This study was the first large population study performed with the aim of analyzing the performance of the combined test in Korea. This study demonstrated that the detection rates and other figures of the first trimester combined test are comparable to the results reported in other papers worldwide. Consequently, if strict conditions for good screening outcomes are achieved, the first trimester combined test might well be the earliest detectable screening, improving detection rates without increasing karyotyping or economic and other implications that inevitably ensue.
Chromosomal anomalies are a leading cause of perinatal mortality and developmental abnormality. Consequently, the principal goal of prenatal testing is to screen for chromosomal anomalies and to provide genetic counseling for parents. The American College of Obstetrics and Gynecology recommended that prenatal test to be offered to all pregnant women [1]. However, invasive genetic screening methods, such as chorionic villi sampling or amniocentesis, are limited to high-risk patients owing to the potential risks for procedure-related pregnancy loss. Until the mid-1980s, maternal age has been the most frequently applied marker for aneuploidy screening. In the 1980s, maternal serum screening and second-trimester ultrasonography were widely utilized. Later, in the 1990s, many studies revealed that maternal age, fetal nuchal translucency (NT), maternal serum free β-human chorionic gonadotropin (hCG) and pregnancy-associated plasma protein-A (PAPP-A) have been associated with aneuploidy and adverse obstetric outcomes [2345]. The "Quad screen", compromising alpha-fetoprotein (AFP), hCG, unconjugated estriol (E3), and inhibin-A, is the most efficient multiple-marker screening test in the second trimester. In addition, there are more options such as integrated, sequential test and cell-free DNA screening. However, first-trimester genetic screening has been one of the most popular screening protocol, and screening powers of the tests are an obstetrical issue until now. Many studies are ongoing to reveal the most sensitive, specific and effective screening tools for use during the first trimester.
There are many strategies available for screening for chromosomal abnormalities including the first trimester combined test, triple test, quadruple test, sequential test and integrated test. Except for the first trimester combined test, all of the others can provide screening results in the second trimester. In the first trimester combined test, the risk is calculated based on the ultrasonographic findings of NT and maternal serum levels of free β-hCG and PAPP-A. First-trimester screening not only allows early reassurance or early diagnosis of aneuploidy, but also provides an option of earlier and safer termination of pregnancy in affected cases. Consequently, the first trimester combined test has become one of the most popular and useful screening strategies. The screening performance of the first trimester combined test has been reported as being up to 82% to 95% detection rate with a 5% to 7% false positive rate [45678910111213].
For second-trimester screening for Down syndrome, different serum markers are utilized. Using the level of AFP, unconjugated E3 and free β-hCG together, the sensitivity and specificity of the triple test are higher than screening with AFP alone [14]. However, when the false-positive rate is fixed at 5% in order to compare the screening performance between the screening tools, the detection rate was found to be 66.8% to 77% with the triple test and 75.9% to 92% with the first trimester combined test. The sensitivity of the triple test was lower than the combined test [5101516]. The quadruple test, which uses the fourth marker, inhibin-A, in addition to the other three markers, has 7% higher sensitivity when applying a fixed 5% false-positive rate [15]. Large studies, such as that conducted by Wald et al. [14] and others, have revealed that when inhibin-A was added to the traditional triple marker test, a detection rate of 83% was achieved, which was 6% higher than the 77% detection rate found with the triple test. This result was similar to that produced with the first trimester combined test.
Edward syndrome (trisomy 18) is the second most common form of chromosomal aneuploidy. The relative proportion of trisomy 21 to trisomy 18 is about three to one at 10 to 14 weeks of pregnancy. The first trimester screening of trisomy 18 is based on the ultrasonographic finding of NT and decreases in maternal serum PAPP-A and free β-hCG. This test can detect 86% to 89% of cases with a 0.5% to 1.0% false-positive rate [17181920].
In Korea, the first trimester combined test has been widely used but little information is available on the performance of this screening method in Korean population. The aim of this study was to examine the performance of the first trimester combined test in the general population of Korea by analyzing the detection rate, false-positive rate and the odds of being affected given positive results in one center, as well as to explore the usefulness by comparing the performance of the test with that of alternatives.
The data for this study were derived from the clinical implementation of the first trimester combined test when screening for aneuploidy at 10+0 to 13+6 weeks of gestation. Patients attended routine antenatal care at the Obstetrics and Gynecology department of Ewha Womans University Mokdong Hospital between January 1st 2008 and December 31st 2012. The first trimester combined test was offered routinely. A total of 1,270 pregnant women underwent the first trimester combined test. Two hundred forty pregnant women who had no records on fetal outcomes were excluded. Twenty five pregnant women who had multiple pregnancies were also excluded. In total, the medical records of 1,005 pregnant women were analyzed.
All women underwent ultrasound examination to estimate gestational age through the measurement of the fetal crown-rump length (CRL). Trans-abdominal ultrasound examination was performed using three pieces of equipment (Accuvix XQ and Accuvix V20, Medison Co., Seoul, Korea; Voluson E8, GE Healthcare, Milwaukee, WI, USA). NT thickness were measured with CRL of 38–84 mm at the time of examination in viable pregnancies. The CRL was obtained by measuring the fetal length from the tip of the cephalic pole to the tip of the caudal pole in the midsagittal plane. The NT thickness was obtained by measuring the maximum thickness of the subcutaneous translucency between the skin and the soft tissue overlying the cervical spine in the midsagittal plane while in the fetal neutral position [2122].
Immediately after the ultrasound examination, maternal blood was sampled using a serum separation tube (4 mL each) for the analysis. The serum was separated by centrifugation and stored at 2℃ to 8℃ until being tested on the following day. The blood samples were delivered to the Neodin Medical Institute (Seoul, Korea). The sample was analyzed by means of fluoroimmunometric assay using an automated AutoDelfia system (Perkin Elmer Brazil, Wallac, Turku, Finland). The values of Free β-hCG and PAPP-A were divided by their respective day-specific median levels and expressed in multiples of the median for each marker. To control for the known increase in the multiples of the median with gestational age, different reference values were employed by the institute. Analysis of NT thickness, PAPPA-A and β-hCG was performed using the HIT-Web program (Hamchoon Inc., Seoul, Korea). The cut-off risk value was 1:250 for Down syndrome and 1:300 for Edward syndrome.
Those with an estimated risk greater than 1 in 250 of carrying a fetus with trisomy 21 or 1 in 300 risk of carrying a fetus with trisomy 18 were offered genetic counseling with the option of an invasive diagnosis test such as chorionic villi sampling or amniocentesis. Routine antenatal care was performed on patients with low risk or those who refused further invasive testing. Follow up on the outcomes of all the pregnancies was performed.
Patient characteristics, test results and pregnancy outcomes were obtained from the ultrasound report and the medical records of Ewha Womans University Mokdong Hospital. The detection rate, false positive rate and odds of being affected given a positive result (OAPR) were calculated in order to evaluate the clinical usefulness of the first trimester combined test in screening for chromosomal abnormalities. The detection rate was calculated as a proportion of affected pregnancies with a positive test result. The false-positive rate was defined as a proportion of unaffected pregnancies with a positive test result. The OAPR was the ratio of the number of affected to unaffected pregnancies with positive results.
Among 1,270 pregnant women who underwent the first trimester combined test between January 1st 2008 and December 31st 2012 at Ewha Womans University Mokdong Hospital, total of 1,005 patients' medical records were analyzed (Fig. 1).
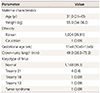
Among the 1,005 pregnancies, no one had a prior pregnancy with chromosome anomaly. The racial origin of the pregnant women was mostly Korean (99.91%) except for one case where the patient was Caucasian (0.09%). The median gestational age at the time of evaluation was 11+4 weeks (range, 10+0 to 13+6 weeks). The median CRL was 49.0 mm (range, 38.0 to 75.3 mm). The median maternal age was 31 years (range, 21 to 43 years). 217 (21.6%) of the women were aged 35 years or older and 497 (49.5%) women were aged between 30 and 34. 291 (29.0%) of the women were aged 29 years or younger. The median maternal weight was 55.0 kg (range, 34 to 96.9 kg) (Table 1).
Of the 1,005 women who underwent first trimester combined test, 92 (9.2%) women had a high-risk result of trisomy 21 with a risk-cutoff of 1:250. Two pregnant women were diagnosed as missed abortion before further invasive testing. After counseling, 26 (27.8%) women declined the offer of an invasive test, while 52 (72.2%) women accepted the offer (Fig. 2). Among these women, four (7.7%) underwent chorionic villi sampling while 48 (92.3%) underwent amniocentesis after 15 competed weeks of gestational age. Fetal death was not reported after invasive testing. In the group of patients who underwent chorionic villi sampling, one case of trisomy 13 and one case of Turner syndrome were identified, while two of the four pregnancies were normal. Among the patients who underwent amniocentesis, trisomy 21 was identified in two cases while 46 of 48 were normal. In the group of women who declined invasive testing, all 26 cases were normal.
In the total screened population, four cases were identified as trisomy 21. Among these, three cases had positive results in the first trimester combined test and were suspected to have trisomy 21. One case was diagnosed as missed abortion before further testing; chromosomal analysis was performed on the abortus afterwards (Table 2, case 1). Diagnosis of trisomy 21 was confirmed by amniocentesis in two cases and the pregnancies were terminated (Table 2; cases 2, 3). In the one case who had a negative result in the screening, heart anomaly (tetralogy of Fallot) and renal abnormality (multicystic dysplastic kidney and pyelectasis) were revealed by antenatal routine ultrasonography. Karyotyping was offered but the patient declined to perform further invasive testing. Postnatal karyotyping revealed trisomy 21 (Table 2, case 4).
In the present report, the first trimester combined test achieved 75.0% sensitivity with a 7.09% false-positive rate. Specificity for trisomy 21 was found to be 92.91%. The positive predictive value was 4.05% and the OAPR was 1:23 (Table 3).
Among the 1,005 pregnant women, 14 (1.4%) women had a high risk of trisomy 18 using a cutoff value of 1:300. Of the 991 cases with a low risk of trisomy 18, chromosomal abnormality was not found. There were two cases of fetal death in utero before further testing (Fig. 3). Among these two women, one had a high risk of both trisomy 21 and 18. Diagnosis of trisomy 21 was achieved by karyotyping of abortus tissue (Table 2, case 1). In the other case, karyotyping was not performed but the fetus was considered as normal because ultrasonographic features and gross appearance of the abortus were normal. The rest of the women (12 of 14) who had positive results in screening underwent invasive diagnostic test (Fig. 3).
Ten women underwent amniocentesis. The karyotype was normal in all cases. Two women underwent chorionic villi sampling. In one case, trisomy 18 was diagnosed and the pregnancy was terminated (Table 4, case 1). The other case had positive result for both trisomy 21 and 18. Chorionic villi sampling revealed Turner mosaicism. Normal spontaneous vaginal delivery was performed at 38 weeks' gestation (Table 4, case 3). The first trimester combined test achieved 100% sensitivity and 98.71% specificity for trisomy 18, with a false-positive rate of 1.29%. The positive predictive value was 7.14% and the negative predictive value was 100%. The OAPR was 1:13.
Seventy nine of 1,005 women had high risk for either trisomy 21 or trisomy 18. Among these women, nine had a positive result for both trisomy 21 and trisomy 18 (Fig. 4). Among the 926 women who had a low risk for both, only one case was revealed to be trisomy 21 postnatally. The rest of 925 cases were normal.
In 79 cases who had a positive result for trisomy 21 or trisomy 18, two cases of other chromosomal abnormalities were diagnosed; one case of Turner syndrome and one case of Patau syndrome (trisomy 13). In both cases, the patients underwent chorionic villi sampling. In the case of Turner syndrome, the patient had high risk for both Down syndrome and Edward syndrome in the first trimester combined test. In the case of trisomy 13, the patient had had a positive result only for trisomy 21. The pregnancy was terminated at 15 weeks (Table 4).
Of the seven pregnancies with chromosomal abnormality, the first trimester combined test correctly identified six cases. In screening for all aneuploidies, the false-positive rate, sensitivity and positive prediction rate were 7.31, 85.71 and 7.59%, respectively. The OAPR was 1:12 (Table 3).
In this study, the false-positive rate for the first trimester combined test for trisomy 21 was 7.09%, which was similar in comparison to 5% to 7% in other studies. It was somewhat higher than the usual set value of 5%. The detection rate was 75%, which is similar to values of 75.9% to 95% produced by other studies [56789101316]. The detection rate was much higher than that of the triple test, which has been found to be 60% to 77% in other countries and 77.8% in South Korea, and was similar to that of the quadruple test [91523]. When compared to the quadruple test, first trimester combined test can achieve a similar detection rate and can provide earlier diagnosis; consequently, it has demonstrated its usefulness in screening for trisomy 21. The OAPR was 1:23, which was similar in comparison to the 1:25 found in the SURUSS (Serum Urine and Ultrasound Screening Study) trial, performed by Wald et al. in 2003 [24]. Additionally, the first trimester combined test has also proved to be reliable on providing accurate low-risk results, based on the specificity of 92.91% and the negative predictive value of 99.89%.
The false-positive rate for the first trimester combined test for trisomy 18 was 1.29%, similar to the 0.5% to 1.0% found in other studies. The combined test detected 1 (100%) of 1 case. Previous studies had recorded 86% to 89% detection rate, thus our study achieved satisfactory result in screening for trisomy 18 [17181920]. The sensitivity, specificity and false-negative rate were 100%, 98.71%, and 0%, respectively, on the basis of which, it has proved its performance for not only trisomy 21 but for trisomy 18 as well, despite the limitation of the small number of evaluated patients.
The first trimester combined test could also be used to screen for chromosomal abnormalities other than trisomy 21 and 18, including trisomy 21 and Turner syndrome. In our study, the test demonstrated an 85.7% detection rate with a 7.31% false-positive rate when considering chromosomal abnormalities altogether, which is higher than the 75% detection rate for trisomy 21 only. Of the seven women exhibiting chromosomal anomalies, there was one case that had produced a false negative result during screening. The rest of six women had a positive result for either Down syndrome or Edward syndrome when using the first trimester combined test; consequently, these women could undergo timely invasive diagnostic testing and genetic counseling. Owing to this, it is suggested that invasive diagnostic testing and genetic counseling are required for all women who demonstrate a positive result in the first trimester combined test, in order to reveal, not only trisomy 21 or trisomy 18, but also other chromosomal abnormalities.
Seventy two percent of the women with a positive screening result agreed to perform further invasive diagnostic testing. 72.2% of the Down syndrome positive and 92.3% of the Edward syndrome positive cases underwent invasive testing. No miscarriages were reported. Complication rate in this study was lower than predicted, compared to a 0.5% to 1% abortion rate which was previously reported by other investigators [25].
In this study, the first trimester combined test was performed between 10+0 and 13+6 weeks of gestation. Both serum markers and NT are affected by the gestational age of the fetus. The discrimination of PAPP-A is greatest at 10 weeks and declines afterwards, whereas screening performance of free β-hCG improves with increasing gestational age until 13 weeks. Also there might be a difference in screening power depending on the gestational age of NT measurement [26]. We performed NT measurement from 10+0 weeks of gestational age and 38 mm of CRL, following Nuchal Translucency Quality Review program. PAPP-A is the most important hormonal parameter which becomes normal at the end of the 14 weeks of gestational age even in trisomy 21 pregnancies. As Schuchter et al. [2] presumed, the higher detection rate of PAPP-A at early stage of screening may compensate for any hypothetical NT weakness in early pregnancy [27].
The patients could obtain the screening result and genetic counseling at one or two weeks following testing. This was not the optimum timing for chorionic villi sampling, considering that chorionic villi sampling is generally performed between 10 and 13 weeks of gestation. Consequently, the patients with positive results tended to have amniocentesis rather than chorionic villi sampling. Despite this issue, the first trimester combined test was still able to provide information earlier in comparison with the second trimester screening tests.
The first trimester combined test has a limitation in diagnosing other conditions such as neural tube defect and gastroschisis owing to the fact that maternal AFP is not useful for screening neural tube defects before 14 weeks of pregnancy, which is the period that the first trimester combined test can be performed [2829]. To overcome this limitation, routine antenatal ultrasonography and a second-trimester anomaly scan were performed to detect any structural anomaly during antenatal care [3031]. One case of neural tube defect was diagnosed by ultrasound. One case with false negative screening result was found to be abnormal with multiple anomaly with follow up ultrasonography, and diagnosed as trisomy 21 postnatally. Considering combined test and ultrasonography altogether, no cases of missed diagnosis were reported in this study.
The limitation of this study is a small sample size in comparison with larger studies. Additionally, because this study was conducted in only one center, the result cannot fully represent the screening performance in all Korean population. As mentioned previously, overall screening performance might be affected by the test timing. The NT was measured by multiple observers; therefore, there might be an error due to interobserver variation. Finally, there were 240 women who were lost to follow up at the center. There might be an error with these women who had no records of fetal outcome.
Recently, cell-free DNA analysis has offered a new diagnostic approach for screening of fetal aneuploidies. There are several studies that attempt to improve the performance of aneuploidy screening by using cell-free DNA as a first-line method or as a contingent on the result of the first trimester combined test. However, the first trimester combined test is still serving a primary role in screening for aneuploidy, because cost-effectiveness should be considered first in order that cell-free DNA may replace the first-line screening method instead of combined test [1332].
In summary, the purpose of this study was to provide information on screening performance of the first trimester combined test in a medical center in Korea and to compare the results with those of the Western countries. To our knowledge, this study is the first large-population study analyzing the result of the first trimester combined test performed in Korea.
This study has proven that the detection rate and other qualities of the first trimester combined test conducted in this medical center are comparable to others that have been reported previously. Consequently, if strict conditions for good screening outcomes are maintained, the first trimester combined test might well be an early screening method that can improve detection rates without increasing invasive test procedures, as well as without increasing the economic and other implications that inevitably follow with testing. It is important to ensure the screening power of tests especially in the time when the cell-free DNA screening test and its role are being debated [16]. As demonstrated in this study, the first trimester combined test is able to provide an effective and reliable screening result with a lower risk of perinatal complication and patient anxiety about diagnostic procedures.
Figures and Tables
Fig. 1
First trimester screening protocol using combined test. NT, nuchal thickness; PAPP-A, pregnancy-associated plasma protein-A; fβ-hCG, free β-human chorionic gonadotrophin.

Fig. 2
Pregnancy outcome according to screening of trisomy (T) 21. CVS, chorionic villous sampling; TS, Turner syndrome.

Fig. 3
Pregnancy outcome according to screening of trisomy (T) 18. CVS, chorionic villous sampling; TS, Turner syndrome.
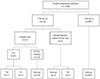
Fig. 4
Pregnancy outcome according to screening of trisomy (T) 21 and 18. CVS, chorionic villous sampling; TS, Turner syndrome.

Table 3
Results of the analyses for the T21 and aneuploidy

T, trisomy; FP, false-positive; FN, false-negative; TP, true-positive; TN, true-negative; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; FPR, false-positive rate; FNR, false-negative rate; DR, detection rate; OAPR, odds of being affected given a positive result.
References
1. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007; 109:217–227.
2. Schuchter K, Hafner E, Stangl G, Metzenbauer M, Hofinger D, Philipp K. The first trimester 'combined test' for the detection of Down syndrome pregnancies in 4939 unselected pregnancies. Prenat Diagn. 2002; 22:211–215.
3. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down's syndrome. Prenat Diagn. 1997; 17:821–829.
4. Spencer K, Spencer CE, Power M, Moakes A, Nicolaides KH. One stop clinic for assessment of risk for fetal anomalies: a report of the first year of prospective screening for chromosomal anomalies in the first trimester. BJOG. 2000; 107:1271–1275.
5. Spencer K, Spencer CE, Power M, Dawson C, Nicolaides KH. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years prospective experience. BJOG. 2003; 110:281–286.
6. Nicolaides KH, Spencer K, Avgidou K, Faiola S, Falcon O. Multicenter study of first-trimester screening for trisomy 21 in 75 821 pregnancies: results and estimation of the potential impact of individual risk-orientated two-stage first-trimester screening. Ultrasound Obstet Gynecol. 2005; 25:221–226.
7. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Semin Perinatol. 2005; 29:225–235.
8. Kagan KO, Wright D, Baker A, Sahota D, Nicolaides KH. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2008; 31:618–624.
9. Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM, et al. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technol Assess. 2003; 7:1–77.
10. Engels MA, Heijboer AC, Blankenstein MA, van Vugt JM. Performance of first-trimester combined test for Down syndrome in different maternal age groups: reason for adjustments in screening policy? Prenat Diagn. 2011; 31:1241–1245.
11. De Biasio P, Siccardi M, Volpe G, Famularo L, Santi F, Canini S. First-trimester screening for Down syndrome using nuchal translucency measurement with free beta-hCG and PAPP-A between 10 and 13 weeks of pregnancy: the combined test. Prenat Diagn. 1999; 19:360–363.
12. Crossley JA, Aitken DA, Cameron AD, McBride E, Connor JM. Combined ultrasound and biochemical screening for Down's syndrome in the first trimester: a Scottish multicentre study. BJOG. 2002; 109:667–676.
13. Lee FK, Chen LC, Cheong ML, Chou CY, Tsai MS. First trimester combined test for Down syndrome screening in unselected pregnancies: a report of a 13-year experience. Taiwan J Obstet Gynecol. 2013; 52:523–526.
14. Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ. 1988; 297:883–887.
15. Cuckle H. Biochemical screening for Down syndrome. Eur J Obstet Gynecol Reprod Biol. 2000; 92:97–101.
16. Baer RJ, Flessel MC, Jelliffe-Pawlowski LL, Goldman S, Hudgins L, Hull AD, et al. Detection rates for aneuploidy by first-trimester and sequential screening. Obstet Gynecol. 2015; 126:753–759.
17. Tul N, Spencer K, Noble P, Chan C, Nicolaides K. Screening for trisomy 18 by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn. 1999; 19:1035–1042.
18. Sherod C, Sebire NJ, Soares W, Snijders RJ, Nicolaides KH. Prenatal diagnosis of trisomy 18 at the 10-14-week ultrasound scan. Ultrasound Obstet Gynecol. 1997; 10:387–390.
19. Spencer K, Mallard AS, Coombes EJ, Macri JN. Prenatal screening for trisomy 18 with free beta human chorionic gonadotrophin as a marker. BMJ. 1993; 307:1455–1458.
20. Biagiotti R, Cariati E, Brizzi L, Cappelli G, D'Agata A. Maternal serum screening for trisomy 18 in the first trimester of pregnancy. Prenat Diagn. 1998; 18:907–913.
21. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10-13 weeks of pregnancy. Prenat Diagn. 1998; 18:281–286.
22. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005; 353:2001–2011.
23. Choi YK, Kim MY, Han JY, Ryu HM, Yang JH, Kim ES, et al. A study about the effectiveness of triple marker test as a screening test for chromosomal aneuploidy. Korean J Obstet Gynecol. 1999; 42:1935–1942.
24. Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003; 10:56–104.
25. Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. 2003; (3):CD003252.
26. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. BJOG. 2004; 111:521–531.
27. Gyselaers WJ, Vereecken AJ, Van Herck EJ, Straetmans DP, de Jonge ET, Ombelet WU, et al. Population screening for fetal trisomy 21: easy access to screening should be balanced against a uniform ultrasound protocol. Prenat Diagn. 2005; 25:984–990.
28. Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992; 304:867–869.
29. Sebire NJ, Spencer K, Noble PL, Hughes K, Nicolaides KH. Maternal serum alpha-fetoprotein in fetal neural tube and abdominal wall defects at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1997; 104:849–851.
30. Johnson SP, Sebire NJ, Snijders RJ, Tunkel S, Nicolaides KH. Ultrasound screening for anencephaly at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1997; 9:14–16.
31. Campbell J, Gilbert WM, Nicolaides KH, Campbell S. Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population. Obstet Gynecol. 1987; 70:247–250.
32. Gil MM, Revello R, Poon LC, Akolekar R, Nicolaides KH. Clinical implementation of routine screening for fetal trisomies in the UK NHS: cell-free DNA test contingent on results from first-trimester combined test. Ultrasound Obstet Gynecol. 2016; 47:45–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download