Abstract
We describe the successful removal of a pelvic contraceptive coil in a symptomatic 46-year-old patient who had Essure devices for four years, using a combined hysteroscopy-laparoscopy-fluoroscopy approach. Following normal hysteroscopy, at laparoscopy the right Essure implant was disrupted and its outer nitinol coil had perforated the fallopian tube. However, the inner rod (containing polyethylene terephthalate) had migrated to an extrapelvic location, near the proximal colon. In contrast, the left implant was situated within the corresponding tube. Intraoperative fluoroscopy was used to confirm complete removal of the device, which was further verified by postoperative computed tomography. The patient's condition improved after surgery and she continues to do well. This is the first report to describe this technique in managing Essure complications remote from time of insertion. Our case highlights the value and limitations of preoperative and intraoperative imaging to map Essure fragment location before surgery.
Several tubal occlusion methods are now available for permanent female contraception, using either a transcervical or transabdominal approach. Hysteroscopic sterilization (HS) is a relatively new technique considered to be safe and effective, and recent trends suggest that patients are preferentially requesting HS over conventional laparoscopic bilateral tubal ligation (BTL). The only Food and Drug Administration-approved method for HS available at present is the Essure procedure (Bayer AG, Leverkusen, Germany). While many women with Essure devices remain asymptomatic and report satisfactory contraceptive efficacy, important issues have been raised regarding this medical device. For example, while HS patients may have a similar risk of contraceptive failure compared to BTL, longitudinal follow-up revealed a more than 10-fold higher risk of requiring subsequent surgery compared with patients undergoing standard BTL [1]. A meta-analysis of all available Essure data (37 published studies as of 2015) found that none were randomized controlled trials, so contraceptive failure rates could not be calculated; likewise the incidence of Essure complications and their severity was impossible to determine [2].
While having an accessible, minimally invasive alternative to surgical BTL is an important and laudable reproductive health objective, there appears to be much about Essure that remains unsettled. The present report contributes additional information about HS by characterizing a symptomatic Essure presentation accompanied by device fragmentation and extrapelvic migration. Moreover, we describe a non-hysterectomy technique for intact laparoscopic removal of a pelvic Essure coil using intraoperative fluoroscopy.
A 46-year-old non-smoking Caucasian G4P2022 presented for evaluation of chronic pelvic pain first noted following HS in July 2011. The Essure procedure was completed under ketorolac intravenous sedation in an ambulatory surgery center setting. Three months later, the patient was informed that bilateral tubal occlusion was confirmed by hysterosalpingogram, but a detailed review of findings was not provided. Soon after HS the patient reported increased, irregular vaginal bleeding and dyspareunia. Within 4 to 6 weeks of her Essure procedure the patient developed intermittent right lower quadrant pain, headaches, insomnia, lower extremity paresthesia, intolerance to cold, and fatigue.
Although the patient was in excellent general health before HS and only visited the doctor for annual check-ups, following the Essure procedure she had approximately 36 clinic appointments (partial year). No specific diagnosis or treatment was offered. Her symptoms persisted during subsequent years and insurance records showed a sharp uptick in frequency of health visits following the Essure procedure: 34 billed encounters in 2012 to discuss pelvic pain issues with various healthcare providers, 22 consultations in 2013, 28 visits in 2014, and 54 appointments in 2015 (partial year). In addition to consultations, these visits included multiple in-office ultrasounds, three computed tomography scans, and blood tests with no abnormal findings identified from these evaluations. The patient obtained a flat panel abdominal X-ray (anterior-posterior view) in May 2015 and this study showed the left Essure implant located within the corresponding fallopian tube, although the right device was in two asymmetric parts.
In September 2015, the patient had a follow-up hysterosalpingogram which showed a normal endometrial cavity and bilateral tubal occlusion. Consistent with the confirmatory hysterosalpingogram performed after HS, the right Essure device appeared disrupted with a small portion possibly corresponding to the medial terminal marker near the right cornu, but the majority of the implant was situated at the lateral pelvis.
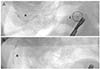
In November 2015, the patient underwent diagnostic hysteroscopy and 5-mm triple-port laparoscopy with partial bilateral salpingectomy for removal of Essure implants. The intrauterine compartment appeared grossly normal and both tubal ostia were visualized. At laparoscopy, both ovaries were normal appearing and the outer coil of the right Essure device could be seen protruding from tubal serosa lateral to the right uterotubal junction (Fig. 1A). This was removed without difficulty using bipolar cautery and circumferential dissection, as previously described [3]. However, a component of the right Essure device was fluoroscopically identified laterally and cephalad to the uterine fundus, although the fragment could not be seen during laparoscopy. The left implant was entirely contained within tubal tissue and was removed using the same technique, with the left implant closely inspected during laparoscopy to verify presence of all terminal markers. Here, it was noted that the inner rod's terminal (medial) marker was absent (Fig. 1B). Intraoperative fluoroscopy was performed and this permitted retrieval of the device remnant (Fig. 2). Postoperative computed tomography was performed which showed the left cornual region was free of any foreign body, but the right Essure fragment was lodged near intestinal serosa at the proximal colon. Because the procedure ended in the late afternoon, the patient stayed overnight and was discharged home from the outpatient surgery floor the following morning. Her postoperative course has been uncomplicated and she continues to do well.
In this report, a case of minimally-invasive surgical management of Essure associated symptoms is described, resulting in substantially ameliorated chronic pain and other problems. While our patient experienced several Essure-associated symptoms which have been noted by others, including device migration [45] and implant fragmentation [6], the surgical approach used here to remove the pelvic coil is uncommon. Indeed, this is the first reported case of laparoscopic retrieval of pelvic coil fragments under fluoroscopic guidance remote from the time of initial hysteroscopic sterilization. Unfortunately, splintering, migration, and impaction of the device near the proximal colon may mean that our patient's journey may not be over if additional surgery is advised to extract the abdominal Essure fragment.
There is no Essure patient registry and frequency of adverse events can only be estimated from number of kits sold, rather than based on absolute number of patients. Women can have up to 5 Essure devices placed [3], so accurate and reliable determination of safety and efficacy awaits further study. In the absence of properly controlled formal studies, the published literature on the Essure device consists of non-randomized clinical trials and case reports [7]. Such work is not without value, and has supplied anecdotal evidence for tissue puncture, device migration, implant fragmentation, and injury to adjacent structures after hysteroscopic sterilization. Most adverse events have not been formally described, as more than 5,000 complications associated with Essure have been uploaded to the US Food and Drug Administration via the MAUDE (Manufacturer and User Facility Device Experience) platform [8].
As Essure is designed and intended for permanent contraception, it is not surprising that a consensus has yet to emerge regarding an optimal surgical approach for its removal. Surgical excision of Essure implants can be facilitated by intraoperative fluoroscopy [79], but not all institutions will have ready access to personnel and equipment necessary to complete this procedure. Literature on fluoroscopy-assisted Essure fragment removal is extremely sparse, and ours is only the second published report detailing this approach with laparoscopy. One case of successful Essure removal eight days after placement with fluoroscopy was reported in 2015 [6]. Fluoroscopy has been successfully used to remove larger non-permanent contraceptive implants imbedded in soft tissue of an extremity [10], but the procedure has not been standardized for removal of Essure devices in the abdomen or pelvis.
Removal of contraceptive coils becomes a more complex operation if the device has fragmented. Some Essure components are radiolucent and will not be visible on a standard radiographs [11] or fluoroscopy, so an awareness of imaging limitations in important. Indeed, for our patient a comparison of intraoperative fluoroscopy views with corresponding intraoperative photographs of the right uterotubal junction illustrates this point. Device retrieval in these situations may require co-management with interventional radiology, and the technical skills needed for removing permanent prostate brachytherapy devices may find cross-application in this gynecological context. Like Essure fragments, radioactive seeds used in permanent prostate brachytherapy can migrate in relation to soft tissue secondary to parenchymal edema or other factors. Brachytherapy treatments occasionally mandate intraoperative modification to allow for radioactive pellet migration, and this provides a surgical challenge comparable to that encountered with Essure fragment removal. In both instances, the inability to reliably and precisely localize the foreign body (either radioactive pellet or Essure shrapnel) represents a major technical challenge [1213]. To address this, the mobile nonisocentric C-arm is the most common intraoperative fluoroscopy instrument available in modern hospitals [13]. The equipment used for our patient was comparable to C-arm cone-beam computed tomography scan (10 mGy to isocenter) used for vascular and orthopedic applications, with ambient radiation exposure between 29 mR (0.26 mSv) at 35 cm from isocenter to <0.5 mR (<0.005 mSv) at 2 m from isocenter. It should be noted that nitinol is a low density alloy and image quality and resolution using C-arm cone-beam computed tomography has not been validated for fragments smaller than approximately 2 mm [14].
Because pelvic and abdominal soft tissue will not be visible on standard fluoroscopy, additional techniques including intraoperative sonography (or, preferentially, intraoperative computed tomography) are required to safely navigate and retrieve very small, low-density objects like a fragmented Essure coil. This approach has been used successfully in orthopedic settings where the target is characterized by significantly greater mass than the nitinol used in Essure.
Considerable patient-level data has demonstrated that Essure migration may involve intestinal adhesions, small bowel obstruction, bowel perforation, or persistent pelvic pain [13]. Management of these Essure-associated complications usually leads to surgical removal of the devices, particularly in the setting of pelvic pain [5]. A multidisciplinary approach with pelvic surgeons, colorectal surgeons, and radiologists may provide improved results for women suffering with Essure-related symptoms. More standardized data reporting in published Essure studies is also recommended.
Figures and Tables
Fig. 1
(A) Laparoscopic view of right adnexa post-Essure placement, showing normal uterine exterior (U) and perforation of right Fallopian tube (R) by nitinol coil fragment. The inner (polyethylene terephthalate) rod had migrated out of the pelvis, lodging near proximal colon (not visualized during laparoscopy). (B) Proximal margin of divided left Fallopian tube (L), with outer nitinol coil (arrow) and inner polyethylene terephthalate rod demonstrating missing terminal marker (circle).

Acknowledgments
The authors wish to thank Rachael L. Lopez, MD (Saddleback Women's Medical Group) for her intraoperative assistance for this case.
References
1. Mao J, Pfeifer S, Schlegel P, Sedrakyan A. Safety and efficacy of hysteroscopic sterilization compared with laparoscopic sterilization: an observational cohort study. BMJ. 2015; 351:h5162.
2. la Chapelle CF, Veersema S, Brolmann HA, Jansen FW. Effectiveness and feasibility of hysteroscopic sterilization techniques: a systematic review and meta-analysis. Fertil Steril. 2015; 103:1516–1525. 1525.e1–1525.e3.
3. Sills ES, Walsh DJ, Jones CA, Wood SH. Endometrial fluid associated with Essure implants placed before in vitro fertilization: considerations for patient counseling and surgical management. Clin Exp Reprod Med. 2015; 42:126–129.
4. Ricci G, Restaino S, Di Lorenzo G, Fanfani F, Scrimin F, Mangino FP. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014; 10:963–968.
5. Rezai S, LaBine M, Gomez Roberts HA, Lora Alcantara I, Henderson CE, Elmadjian M, et al. Essure microinsert abdominal migration after hysteroscopic tubal sterilization of an appropriately placed Essure device: dual case reports and review of the literature. Case Rep Obstet Gynecol. 2015; 2015:402197.
6. Braginsky L, George ST, Locher SR. Management of perforated essure with migration into small and large bowel mesentery. J Minim Invasive Gynecol. 2015; 22:504–508.
7. Dhruva SS, Ross JS, Gariepy AM. Revisiting Essure: toward safe and effective sterilization. N Engl J Med. 2015; 373:e17.
8. US Food and Drug Administration. MAUDE adverse event report: Bayer Healthcare LLC Essure device, occlusion, tubal, contraceptive [Internet]. Silver Spring (MD): US Food and Drug Administration;2015. cited 2015 Dec 15. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=4531887.
9. Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012; 19:667–670.
10. Guiahi M, Tocce K, Teal S, Green T, Rochon P. Removal of a Nexplanon implant located in the biceps muscle using a combination of ultrasound and fluoroscopy guidance. Contraception. 2014; 90:606–608.
11. Guelfguat M, Gruenberg TR, Dipoce J, Hochsztein JG. Imaging of mechanical tubal occlusion devices and potential complications. Radiographics. 2012; 32:1659–1673.
12. Nag S, Ciezki JP, Cormack R, Doggett S, DeWyngaert K, Edmundson GK, et al. Intraoperative planning and evaluation of permanent prostate brachytherapy: report of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2001; 51:1422–1430.
13. Kuo N, Dehghan E, Deguet A, Mian OY, Le Y, Burdette EC, et al. An image-guidance system for dynamic dose calculation in prostate brachytherapy using ultrasound and fluoroscopy. Med Phys. 2014; 41:091712.
14. Daly MJ, Siewerdsen JH, Moseley DJ, Jaffray DA, Irish JC. Intraoperative cone-beam CT for guidance of head and neck surgery: assessment of dose and image quality using a C-arm prototype. Med Phys. 2006; 33:3767–3780.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download