Abstract
Female adnexal tumor of probable Wolffian origin (FATWO) is a rare disease entity that arises from the mesonephric duct system. FATWO is different than other gynecological cancers in terms of embryology. Here, we describe the case of a 52-year-old woman with malignant FATWO. The patient underwent explorative laparotomy and surgical staging after a frozen section revealed malignancy. Detailed examination of the pathologic findings were consistent with FATWO. Counseling and further testing were provided to the patient to assess the risk of germline mutation and epigenetic change. An O-6-methylguanine-DNA methyltransferase gene methylation test was positive, and all other tests were normal. This is the first study to report a case of O-6-methylguanine-DNA methyltransferase methylation with FATWO in Korea.
Few studies have been published on female adnexal tumors of probable Wolffian origin (FATWO) since the first report in 1973 by Kariminejad and Scully [1]. The mesonephric (Wolffian duct) system traverses numerous female reproductive organs, including the broad ligament, mesosalpynx, fallopian tubes, ovaries, and peritoneum [2 3], and is believed to be the origin of FATWO. During human embryogenesis, the mesonephric duct is a paired organ that develops into the male reproductive system. In females, with the absence of the anti-Müllerian hormone, the mesonephric duct degenerates and forms the broad ligament, lateral walls of the cervix, and the vagina and uterine corpus [4].
Little is known about the carcinogenesis of FATWO due to its rarity. However, as in other gynecological cancers, both genetic and epigenetic changes (DNA methylation, histone modifications, and non-coding RNAs) in gene expression may contribute to FATWO carcinogenesis [5]. There are currently 80 reported cases of FATWO. Among them, only 21 cases were found to have malignant potential, such as metastasis or recurrence [6]. Here, we report one case of malignant FATWO with epigenetic changes in O-6-methylguanine-DNA methyltransferase (MGMT) methylation.
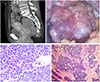
A 52-year-old Korean woman was referred to Samsung Changwon Hospital from a local clinic with a right adnexal mass. She was suffering from a mild pelvic pain for a month. Abdominal and pelvic computed tomography (CT) scans showed an 8-cm solid mass in the left paraovarian area of the posterior uterine wall (Fig. 1A), suggesting uterine leiomyoma or an ovarian tumor. The levels of serum CA 19-9 was 5.92 U/mL and CA 125 was 12.38 U/mL, which are within normal ranges.
A pelviscopy indicated that the 8-cm-sized solid and papillary mass was located in the ovary hilus to the paraovarian area (Fig. 1B). It was diagnosed by frozen biopsy as a malignancy with frequent mitosis (Fig. 1C) with a chance of metastasis to the ovary and adnexa. The patient underwent complete surgical staging, including a total abdominal hysterectomy, bilateral salpingo-oophorectomy, total omentectomy, washing cytology, and pelvic lymph node dissection. During the surgery, an esophagogastroduodenoscopy and colonoscopy were performed to rule out metastatic tumors. A positron emission tomography-CT scan after recovery showed no evidence of distant metastasis.
The pathology results indicated that the left ovary and salpinx contained malignant FATWO (Fig. 1C, D). The tumor displayed cystic and adenomatoid pattern with colloid like material under the microscope. The nuclei showed frequent mitosis. And electron miscoscope showed favor malignant FATWO than mesothelioma with absence of characteristic micovillus suggesting mesothelioma. There was no lymph node metastasis. Immunohistochemistry analysis showed that the ovarian tissue stained focal positive for D2-40, calretinin, CK, CD10, vimentin, CD56, CK7, and mucicarmine, but negative for CK 5/6, ER, EMA, HMP45, chromogranin, synaptophysin, and CK20. Ki 67 staining showed an increased proliferation index of 20% to 30%. Genetic counseling and tests were provided for the patient to assess the risk of germline mutation and epigenetic change. A blood sample was subjected to genetic testing, and no mutations were detected in the BRCA1 or BRCA2 genes. An MGMT gene methylation test was performed and was positive (Fig. 2). The patient was followed up with CT every 3 months. After 9 months, 2.5-cm-sized enhancing nodule at the right side of cul-de-sac area was discovered which was proven to be a local recurrence by positron emission tomography-CT thereafter. The patient received three cycles of chemotherapy with paclitaxel and carboplatin. After three sessions of chemotherapy, the tumor size increased to 2.8 cm. Moreover, there appeared new metastatic nodule at the left side of the cul-de-sac and hepatic tip area. We planned additional debulking surgery and chemotherapy, but she was lost for follow-up.
FATWO is a rare disease. Therefore, its development and tumorigenesis has not been adequately investigated with regard to epigenetic mutation changes. Although FATWO generally has a low malignant potential, some cases of FATWO have been associated with recurrence or metastasis. Of 80 cases reported until now, only 21 cases showed evidence of recurrence. The site of recurrence was liver, pelvis, appendix, peritoneum and omentum. Liver was the most common site of recurrence. In our case, the first recurrence was in the vaginal stump, and second recurrence site was liver surface. There is not currently established chemotherapy regimen to FATWO due to its rarity. There isn't currently established chemotherapy regimen to FATWO due to its rarity. Malignant FATWO does not respond to conventional treatment. The most commonly used regimen was paclitaxel and carboplatin. Gliveec was used in some cases with c-kit positive patients [6]. This is the first FATWO case to study MGMT methylation mutation changes in Korea.
FATWO has a different embryology than other gynecologic malignancies. During female reproductive organ development, the Wolffian duct guides caudal growth of the Müllerian duct. Müllerian ducts, Wolffian ducts, and the urogenital sinus fuse to form a sinovaginal bulb that develops into the lower portion of the vagina [7]. The absence of anti-Müllerian hormone in females causes the Wolffian ducts to degenerate and form the broad ligament, lateral walls of the cervix, and the vagina and uterine corpus. FATWO arises in these remnants. Therefore, FATWO is usually found in the upper region of the Wolffian system, whereas mesonephric adenocarcinomas are found in the lower section [4]. In this case, the tumor arose from the broad ligament.
FATWO is distinguished from well-differentiated endometrioid ovarian adenocarcinomas, endometrioid adenocarcinomas of the fallopian tube, and Sertoli-Leydig cell tumors [3]. FATWO usually arises within the broad ligament, whereas endometrioid adenocarcinomas arise from the fallopian tube [1]. Nuclear atypia is mild to moderate in endometrioid adenocarcinoma, which is more impressive than in FATWO. There is a strong morphological similarity between Sertoli-Leydig cell tumors and FATWO. However, FATWO tends not to demonstrate the endocrine symptoms that are features of Sertoli-Leydig cell tumors such as defeminization and progressive masculinization. Moreover, the absence of Leydig cells helps us diagnose FATWO. Microscopically, FATWO have well-differentiated epithelial cells with tubular, sieve-like, diffuse patterns of growth.
FATWO is characterized by a variety of epithelial patterns. Although it is difficult to confirm its malignancy due to the limited number of reported cases, FATWO with high mitotic activity, cellular atypia, and necrosis usually behave in an aggressive manner [8]. Although none of the previous studies defined a strong correlation between immunohistochemistry and FATWO, one study suggested that the tumors are generally cytokeratin- and vimentin-positive and epithelial membrane antigen (EMA)-negative [9]. In our case, the tumor displayed cystic and adenomatoid patterns in the low-power field and frequent mitotic figures in the high-power field (Fig. 1C, D). Upon immunohistochemical analysis, the tumor showed focal positive staining for D2-40, calretinin, CK, CD10, vimentin, CD56, CK7, and mucicarmine. The tumor was negative for CK 5/6, ER, EMA, HMP45, chromogranin, synaptophysin, and CK20. Ki 67 staining showed an increased proliferation index of 20% to 30%. These findings were consistent with malignant FATWO.
Although it is difficult to assume its malignancy due to limited number of cases, FATWO with high mitotic activity, cellular atypia, and necrosis usually behave in an aggressive manner. The pathophysiologic backgrounds of carcinogenesis of ovary carcinomas and FATWO are not fully understood, but development of genomic instability is believed to be linked with tumorigenesis [10]. This process is extremely complex and includes epigenetic changes that modify gene expression without changing the DNA sequence via DNA hypermethylation, histone modification, adenosine triphosphate-dependent chromatin remodeling, or non-coding RNAs [11]. MGMT is a tumor suppressor that repairs damaged DNA by removing an alkyl group from O-6-guanine [12]. MGMT inactivation in some human cancers is due to hypermethylation of the MGMT promotor region [13].
Hypermethylation of the MGMT promotor has been reported in several studies of gynecological cancers [14]. In our case, the tumor was positive for MGMT promotor methylation. Thus, MGMT inactivation via hypermethylation of the gene promotor may have contributed to the tumorigenesis of this case of FATWO. Although its contribution has been identified in other cancers, further studies are needed to determine the role of MGMT methylation in FATWO. However, since MGMT hypermethylation is associated with ovarian cancer, we hypothesize that it contributes to the carcinogenesis of malignant FATWO. As some cases of FATWO shows malignant behavior and recurrence, our study is a first step to investigate the differentiation of malignant and benign FATWO.
Figures and Tables
References
1. Kariminejad MH, Scully RE. Female adnexal tumor of probable Wolffian origin: a distinctive pathologic entity. Cancer. 1973; 31:671–677.
2. Tamiolakis D, Anastasiadis P. Metastatic female adnexal tumour of probable Wolffian origin: a histocytopathological correlation. Cytopathology. 2007; 18:264–266.
3. Heatley MK. Is female adnexal tumour of probable wolffian origin a benign lesion? A systematic review of the English literature. Pathology. 2009; 41:645–648.
4. Marquette A, Moerman P, Vergote I, Amant F. Second case of uterine mesonephric adenocarcinoma. Int J Gynecol Cancer. 2006; 16:1450–1454.
5. Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999; 286:481–486.
6. Deshimaru R, Fukunaga T, Sato T, Morinaga S, Takahashi M. A case of metastatic female adnexal tumor of probable Wolffian origin. Gynecol Oncol Rep. 2014; 10:22–24.
7. Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation. 2011; 82:117–126.
8. Harada O, Ota H, Takagi K, Matsuura H, Hidaka E, Nakayama J. Female adnexal tumor of probable wolffian origin: morphological, immunohistochemical, and ultrastructural study with c-kit gene analysis. Pathol Int. 2006; 56:95–100.
9. Tiltman AJ, Allard U. Female adnexal tumours of probable Wolffian origin: an immunohistochemical study comparing tumours, mesonephric remnants and paramesonephric derivatives. Histopathology. 2001; 38:237–242.
10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
11. Gerhauser C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr Opin Clin Nutr Metab Care. 2013; 16:405–410.
12. Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res. 2006; 12:328–331.
13. Watts GS, Pieper RO, Costello JF, Peng YM, Dalton WS, Futscher BW. Methylation of discrete regions of the O6-methylguanine DNA methyltransferase (MGMT) CpG island is associated with heterochromatinization of the MGMT transcription start site and silencing of the gene. Mol Cell Biol. 1997; 17:5612–5619.
14. Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006; 6:212.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download