Abstract
Endometrial stromal sarcoma (ESS) is a rare malignancy. Development of extrauterine ESS form endometriosis is particularly rare. The majority of extrauterine ESS occurs in areas with preexisting endometriosis. The most common site is the ovary. We experienced a case of ESS of the ovary that arose from endometriosis with multiple disseminated lesions. This disease was managed by total abdominal hysterectomy, bilateral salpingo-oophorectomy, both pelvic lymph nodes dissection, omentectomy, and appendectomy followed by postoperative high-dose progesterone therapy. Here, we report this case with literature review.
Endometrial stromal sarcoma (ESS) is a rare form of uterine sarcoma. ESS comprises approximately 0.2% of all uterine malignancies and 10% to 15% of all uterine sarcomas [12]. ESS is a malignant tumor that originates from invasive proliferation of cells that resemble stromal cells of normal proliferative endometrium [23]. Predominantly, ESS arises from the uterine corpus. It can also originate from extrauterine sites. The majority of extrauterine ESS arises from endometriosis through a process of malignant transformation [14]. This is an unusual event, only occurring in 0.1% to 0.7% of cases. The most common types of malignant tumors that originate from endometriosis are endometrioid adenocarcinoma and clear cell tumors. ESS is extremely uncommon [5].
ESS arising from endometriosis is considered as an indolent tumor with excellent prognosis. However, late recurrence and distant metastases may occur more than 25 years after its initial diagnosis [6]. Treatment for a disseminated disease is particularly problematic. Targeted therapy for ESS arising from endometriosis remains unclear. However, some studies have reported that adjuvant hormonal therapy is effective for advanced-stage ESS [78].
Here we describe a case of ovarian ESS with multiple metastases derived from pathologically confirmed endometriosis. We managed it with complete surgical resection and hormonal therapy.
A 40-year-old para 1 woman without significant medical history was transferred to Chonnam National University Hospital. She had bilateral ovarian tumors detected by pelvic ultrasonography at a local gynecologic clinic for the evaluation of dysmenorrhea and hypermenorrhea. A pelvic ultrasonography revealed a 6.4×5.8×5.3-cm3 mass with irregular margin in the right ovary and a 6.3×5.2×4.2-cm3 multi-septated cystic mass in the left ovary. There was no obvious ascite in the abdominal cavity. Pulsed Doppler examination revealed low-resistant blood flow (resistance index 0.24). On physical examination, severe adhesion between these tumors and the cul-de-sac was noted. The patient's preoperative CA 125 level was 97.3 U/mL and other tumor markers (CA 19-9, CA 72-4, and CEA) were within normal ranges. Pelvic magnetic resonance imaging showed a 6.0×4.8×5.3-cm3 ill-defined, heterogenous signal intensity mass, including mixed cystic and solid portions in the right ovary. This mass was considered as a malignancy (i.e., clear cell carcinoma) likely arising from underlying endometriosis. Between the posterior portion of the right ovarian tumor and the cul-de-sac, there was a 5.5×5.0-cm2 enhanced solid mass with an irregular margin invading into the rectal serosa (Fig. 1A). Additionally, there was a 7.2×5.9×6.1-cm3 multi-septated cystic mass in the left ovary (Fig. 1B) that was considered as a benign mucinous cystadenoma. Positron emission tomography-computed tomography revealed a high probability of ovarian malignancy involving both ovaries and possibly peritoneal carcinomatosis. The patient's preoperative body mass index was 30.84 kg/m2 (height 158-cm, weight 77 kg).
The patient underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, both pelvic lymph nodes dissection, omentectomy, and appendectomy through median incision at supine position. We performed operation for the basis of epithelial ovarian malignancy. A small number of ascites (500 mL) was present and peritoneal cytology was done. The right ovarian tumor measured at approximately 5.0×5.0-cm2 contained a 2.0×3.0-cm2 solid mass and chocolate-colored fluid. This mass was fixed in the cul-de-sac. It was hard indurated because of cancer infiltration. The left ovary mass measured at roughly 5.0×6.0-cm2 was an inflammatory cyst containing yellowish fluid. Multiple seeding lesions were found at rectal serosa, posterior uterine serosa, and appendix. Multiple whitish nodular lesions of up to 3.0×3.0-cm2 were detected at the omentum. These suspicious lesions were biopsied. No intrauterine tumor was found (Fig. 2E). Intra-operative frozen biopsy analysis of the right ovarian tumor showed ESS with endometriosis because proliferative stromal cells with scant nuclear pleomorphism and mitosis were seen. Postoperative course was complicated by a disrupted vaginal stump. There was no other complication.
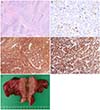
Final pathology was consistent with bilateral ovarian ESS arising from foci of endometriosis and involving the uterine serosa, rectal serosa, parametrium, peritoneum, omentum, and appendix (International Federation of Gynecology and Obstetrics [FIGO] stage IIIC). No evidence of tumor was found in the corpus of the uterus or the uterine cavity. Both pelvic lymph nodes were negative for tumor cells. The result of peritoneal cytology was negative for malignant cells. The mitotic activity in the spindle cells in the cellular area was limited to a maximum of two figures per 10 high-power fields. Stromal tissues had the characteristic tongue-like growth pattern (Fig. 2A). Immunohistochemical staining revealed that neoplastic cells were immunopositive to antibodies specific for cluster of differentiation 10 (CD 10) (Fig. 2C), Ki 67 (Ki index 3% to 5%) (Fig. 2B), and progesterone receptor (Fig. 2D). However, these neoplastic cells were immunonegative to estrogen receptor, desmin, alpha inhibin, calretinin, and actin.
The patient's CA 125 level was decreased from 97.3 U/mL before surgery to 38.8 U/mL after surgery. Adjuvant hormonal therapy was started one month after the operation in an effort to suppress the growth of any microscopic tumors. The patient had been taking oral megestrol acetate (Megesia, Dalimpharm, Seoul, Korea) at 160 mg daily for one month. She complained about weight gain. Therefore, the patient had been taking oral medroxyprogesterone acetate (Farlutal, Pfizer, New York, NY, USA) at 500 mg daily for four months. Six months after the beginning of treatment, her CA 125 level was decreased to 22.7 U/mL. Subsequent Positron emission tomography-computed tomography revealed no evidence of recurrence or metastases. However, she had complaints of generalized edema. Therefore, we decreased her drug dosages. She was taking oral medroxyprogesterone acetate (Provera, Pfizer) at 200 mg daily for three months. Three months later, she refused further hormonal therapy because of lasting side effects. At that time, an abdominopelvic computed tomography showed no evidence of recurrence. Therefore, we stopped the hormonal therapy and planned regular checkups with pelvic examination, tumor markers, and imaging studies. To date, she has shown no signs of recurrence.
ESS arising from foci of endometriosis is extremely rare [12]. In the English literature, the sites of extrauterine ESS include the ovaries, fallopian tubes, broad ligament, vagina, rectosigmoid, intestine, peritoneum of pelvic cavity, mesentery, and liver, with ovary as the most common primary site [367]. The criteria for a diagnosis of ESS arising from endometriosis are clear existence of endometriosis in close proximity to a tumor, no other primary site of a tumor, and a histological appearance compatible with the origin of endometriosis [7]. Our case satisfied all these criteria.
The major microscopic features of ESS are diffuse growth pattern of small and round cells with scant cytoplasm resembling normal proliferative endometrial stromal cell, extraovarian intravascular growth, pericellular reticulum, and fibromatous components [910]. Immunohistochemical staining studies have revealed its reactivity to antibodies specific for vimentin, estrogen receptor, progesterone receptor, and CD 10, but not immunoactive to antibodies of muscle-specific actin or cytokeratin [71011]. Our case was only immunoactive to progesterone receptor, CD 10, and Ki 67 markers of cell proliferation.
In the past, ESSs had been divided into two categories (low-grade or high-grade) based on the number of mitotic figures per 10 high-power fields. However, in the latest 2003 World Health Organization classification, endometrial stromal tumors are divided into endometrial stromal nodules, ESSs (referred to as low-grade ESS in the past), and undifferentiated or high-grade endometrial sarcomas (referred to as high-grade ESS in the past). High-grade or undifferentiated endometrial sarcoma may not originate from the endometrial stroma. They have poor prognosis [812]. ESS has an indolent clinical course with good prognosis. It is rarely disseminated [812]. However, more cases of in primary extrauterine ESS have extended to the extrauterine or the extrapelvic space compared to primary uterine ESS [368]. In a review by Leath et al. [3], among 72 primary uterine ESSs, only one-third have disseminated at diagnosis. In contrast, primary ovarian ESS arising from endometriosis might be more prone to disseminating beyond its site of origin at diagnosis. In a study of 43 cases of extrauterine ESS [6], 76.9% (10/13) of patients with tumors arising from the ovary had disease extension, including 61.5% (8/13) with abdominal extension and 15.4% (2/13) with more distant extensions. High frequencies of disease dissemination were also found in patients with tumors arising from other uncommon sites. Our patient with ovarian tumors originating from endometriosis also presented multiple metastases (extension to tubes, uterine serosa, rectal serosa, parametrium, peritoneum, omentum, and appendix) at the time of diagnosis (FIGO stage IIIC). Late recurrence and distant metastasis may occur in advanced-stage disease more often than that in early stages [4]. Thus, adjuvant therapy following initial treatment may be needed for advanced-stage extrauterine ESS.
Total abdominal hysterectomy with bilateral salpingooophorectomy is considered as the standard management for uterine ESS, although treating both extrauterine ESS and disseminated disease is controversial. Nonetheless, complete surgical cytoreduction without residual disease is considered as the primary therapeutic approach and the most effective treatment for patients with advanced-stage extrauterine ESS [4681113]. Options for adjuvant therapy following surgery such as radiotherapy and chemotherapy are not certain or proven to be effective [3111415]. However, previous studies have reported that hormonal therapy is effective for advanced-stage ESS [78]. Because ESS is mostly steroid receptor-positive tumors, hormonal therapy including progestins (megestrol or medroxyprogesterone), aromatase inhibitors (third generation), and gonadotropin-releasing hormone agonists have been considered as effective in reducing the risk of a recurrence. The presence of estrogen receptors and progesterone receptors may be associated with susceptibility to hormonal
In summary, our case illustrated the findings of a primary ovarian ESS with multiple metastases arising from endometriosis confirmed by typical histologic features after complete surgical resection. This disease was advanced-stage ESS (FIGO stage IIIC). Thus, adjuvant hormonal therapy was begun to suppress the growth of any microscopic tumor. The patient has no evidence of disease at fourteen months after the initial management. Because ESS can recur after a number of years after initial diagnosis, long-term follow-up is advised. This case report shows that adjuvant hormonal therapy could be successful for rare advanced staged ESS, thus providing useful information to better inform and optimize treatment strategies for advanced stage ESS.
Figures and Tables
Fig. 1
Pelvic magnetic resonance imaging finding. (A) A 6.0×4.8×5.3-cm3 ill-defined, heterogeneous signal intensity mass, including mixed cystic and solid portions in the right ovary. This mass was considered as a malignancy (i.e., clear cell carcinoma). Between the posterior portion of the right ovarian tumor and the cul-de-sac, there was a 5.5×5.0-cm2 enhanced solid mass with an irregular margin invading into the rectal serosa. (B) A 7.2×5.9×6.1-cm3 multi-septated cystic mass in the left ovary that was considered as a benign mucinous cystadenoma.

Fig. 2
Microscopic, immunohistochemical, and macroscopic finding. (A) Stromal tissue had the characteristic tongue-like growth pattern (H&E, ×40). (B) Positive for Ki 67 (Ki index 3% to 5%, ×400). (C) Positive for cluster of differentiation 10 (×200). (D) Positive for progesterone receptor (×400). (E) The right ovary, which was measured roughly 5 cm, contained a 3 cm solid mass and chocolate-colored fluid. There was no intrauterine tumor.
References
1. Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary extrauterine endometrial stromal neoplasms: a clinicopathologic study of 20 cases and a review of the literature. Int J Gynecol Pathol. 1993; 12:282–296.
2. Diesing D, Cordes T, Finas D, Loning M, Mayer K, Diedrich K, et al. Endometrial stromal sarcomas: a retrospective analysis of 11 patients. Anticancer Res. 2006; 26:655–661.
3. Leath CA 3rd, Huh WK, Hyde J Jr, Cohn DE, Resnick KE, Taylor NP, et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol. 2007; 105:630–634.
4. Geas FL, Tewari DS, Rutgers JK, Tewari KS, Berman ML. Surgical cytoreduction and hormone therapy of an advanced endometrial stromal sarcoma of the ovary. Obstet Gynecol. 2004; 103(5 Pt 2):1051–1054.
5. Mourra N, Tiret E, Parc Y, de Saint-Maur P, Parc R, Flejou JF. Endometrial stromal sarcoma of the rectosigmoid colon arising in extragonadal endometriosis and revealed by portal vein thrombosis. Arch Pathol Lab Med. 2001; 125:1088–1090.
6. Lan C, Huang X, Lin S, Cai M, Liu J. Endometrial stromal sarcoma arising from endometriosis: a clinicopathological study and literature review. Gynecol Obstet Invest. 2012; 74:288–297.
7. Alcazar JL, Guerriero S, Ajossa S, Parodo G, Piras B, Peiretti M, et al. Extragenital endometrial stromal sarcoma arising in endometriosis. Gynecol Obstet Invest. 2012; 73:265–271.
8. Rauh-Hain JA, del Carmen MG. Endometrial stromal sarcoma: a systematic review. Obstet Gynecol. 2013; 122:676–683.
9. Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Armed Forces Institute of Pathology. Atlas of tumor pathology. 3rd ed. Washington, DC: Armed Forces Institute of Pathology;1998. p. 132–140.
10. Oliva E, Egger JF, Young RH. Primary endometrioid stromal sarcoma of the ovary: a clinicopathologic study of 27 cases with morphologic and behavioral features similar to those of uterine low-grade endometrial stromal sarcoma. Am J Surg Pathol. 2014; 38:305–315.
11. Kim JY, Hong SY, Sung HJ, Oh HK, Koh SB. A case of multiple metastatic low-grade endometrial stromal sarcoma arising from an ovarian endometriotic lesion. J Gynecol Oncol. 2009; 20:122–125.
12. Oliva E, Clement PB, Young RH. Endometrial stromal tumors: an update on a group of tumors with a protean phenotype. Adv Anat Pathol. 2000; 7:257–281.
13. Thomas MB, Keeney GL, Podratz KC, Dowdy SC. Endometrial stromal sarcoma: treatment and patterns of recurrence. Int J Gynecol Cancer. 2009; 19:253–256.
14. Bodner K, Bodner-Adler B, Obermair A, Windbichler G, Petru E, Mayerhofer S, et al. Prognostic parameters in endometrial stromal sarcoma: a clinicopathologic study in 31 patients. Gynecol Oncol. 2001; 81:160–165.
15. Barney B, Tward JD, Skidmore T, Gaffney DK. Does radiotherapy or lymphadenectomy improve survival in endometrial stromal sarcoma. Int J Gynecol Cancer. 2009; 19:1232–1238.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download