Abstract
Placental chorioangioma is a benign non-trophoblastic tumor of the placenta that can have various adverse effects on the mother and fetus depending on its size. Chorioamniotic membrane separation is rare condition of detachment between the amniotic membrane and chorionic membrane. Chorioamniotic membrane separation after the second trimester of pregnancy is usually occurs after invasive procedures or may occur spontaneously; it is mostly associated with fetal abnormalities. Here, we report a case of chorioamniotic membrane separation that might be occurred caused by the seromucinous secretion from a placental chorioangioma.
Placental chorioangioma is a rare and benign non-trophoblastic tumor of the placenta originating from primitive chorionic mesenchyme [1]. Most of these tumors are small and asymptomatic; however, large tumors can occur and these have side effects on both, the mother and fetus, such as fetal anemia, hydrops, polyhydramnios, preterm delivery, fetal growth restriction and fetal demise [234].
Chorioamniotic membrane separation (CMS) is rare condition of detachment between the amniotic membrane and chorionic membrane. CMS may cause premature rupture of membrane, preterm delivery, amniotic band syndrome, or strangulation of the umbilical cord. In severe cases, it can lead to fetal distress and intrauterine fetal death [5]. It can occur after invasive fetal procedures such as fetal surgery and amniocentesis, or it may occur spontaneously; spontaneously occurring CMS is associated with fetal abnormalities [6].
Here, we report a case of a large placental chorioangioma with cystic changes within the tumor and profuse seromucinous fluid collection, which was possibly associated with the cause of CMS.
A 29-year-old woman (gravida 2 and para 1) was referred to our fetal-maternal division at 30+4 weeks of gestation for evaluation of polyhydramnios. There was no remarkable personal and familial history. Ultrasound examination revealed a single fetus with vertex presentation and growth within the normal range for gestational age (1,774 g, 60th percentile). There was no evidence of fetal hydrops or other fetal structural abnormalities. There was polyhydramnios and amniotic fluid index was 58 cm. The placenta was anteriorly placed in the uterus. We found a 4.84×6.33-cm-sized mixed solid and cystic mass with high vascularity within the left lateral placenta and suspected it as a placental chorioangioma (Fig. 1A). The patient was admitted due to abdominal distension and dyspnea. On the 1st hospital day, amnioreduction (approximately 2,000 mL) was performed to alleviate her symptoms. After amnioreduction, the patient's symptoms improved, but she suffered from preterm labor caused by polyhydramnios and was administered tocolytic therapy. During hospitalization, there were no changes in the size or sonographic features of the tumor. Amniotic fluid index ranged from 42 to 54 cm. At 32+3 weeks gestation, she complained of sudden abdominal pain. Her abdomen was much distended, but there was no tenderness or rebound tenderness on the abdomen and no vaginal bleeding or leakage of amniotic fluid. We performed an ultrasound examination and found extensively hypoechoic subchorionic lesion with a depth of 6 cm between the placental membrane and uterus (Fig. 1B). The extensive subchorionic fluid collection was located beside the highly vascular placental tumor. We could not rule out placenta abruption or subchorionic hemorrhage due to vascular rupture of the tumor or CMS. We performed an emergency cesarean section. When we opened the uterus, profuse seromucinous fluid was found outside of the placental membrane (Fig. 1C).
A healthy female neonate was born, weighing 2,000 g with an Apgar score of 7/8. After delivery of the placenta, a solid yellowish tumor was found on the maternal surface and a yellowish cyst below the fetal membrane (Fig. 1D). The contents of the cyst were yellow mucinous or serous material. There was extensive separation of the fetal membranes with the accumulation of mucinous or serous cystic fluid. The size of the separated portion was 26.0×19.5 cm.
The placenta was 22.5×19.5×3.5 cm in dimension, and showed a relatively circumscribed solid mass, measuring up to 6.0 cm in diameter and multiple cystic spaces filled with yellowish fluids under the chorioamniotic membrane, accompanying separation of membranous tissue. The mass revealed diffuse proliferation of small-sized vessels, with large areas of cystic or myxoid degeneration and calcification, especially under the chorioamniotic membranous tissue (Fig. 2). The patient was discharged without any postoperative complications. After several weeks in the hospital, the infant was discharged without any problems of preterm birth.
Placental chorioangioma is more prevalent than other nontrophoblastic tumors of the placenta. Chorioangiomas show variable histological features; common features are angiomatous, cellular, and degenerate [7]. Degenerative chorioangioma displays myxomatous changes or hyaline degeneration and is accompanied by calcification. If chorioangioma has the gross myxomatous changes, it can incorporate Wharton jelly-like material [8]. But there are no reports of placental tumors or chorioangiomas which contain a large volume of mucinous or serous fluid.
Small sized chorioangiomas are usually asymptomatic, and therefore, are usually only noticed after delivery through placental biopsy. On the contrary, the presence of large tumors (≥5 cm) may result in maternal and fetal complications, which include polyhydramnios, preterm labor, fetal congestive heart failure, anemia, and intrauterine fetal death [234]. For chorioangiomas that induce complications, various treatments are available. However, there is a limit. The complications that can occur in the fetus and the mother are dependent on the size and distance of the tumor and the cord insertion of the tumor. In our case, polyhydroamnios and associated preterm labor occurred. Polyhydroamnios complicates 1% to 2% of all pregnancies and underlying etiology of polyhydroamnios include diabetes, various congenital anomalies, multifetal gestation, and placental tumor like chorioangioma. The mechanism of chorioangioma-induced polyhydroamnios is not obvious, but large size tumor can cause obstruction of blood flow and increase intravascular pressure in placenta and it can make transudation into amniotic cavity [9].
CMS is a phenomenon in which the two membranes separate. During early pregnancy, the amnion and chorion are separated. They become fused at 13 to 14 weeks of pregnancy. CMS is not considered a normal phenomenon after the second trimester. Spontaneous CMS may occur but its incidence is rare and has an association with fetal malformations or aneuploidy [6]. We also experienced spontaneous CMS caused by fetal restrictive dermopathy [10]. Most cases of CMS occurred after invasive intrauterine procedures such as amniocentesis, fetoscopy and fetal surgery [5]. Lewi et al. [6] reported that invasive intrauterine procedures caused CMS in most cases (89%) and the amniocentesis was the caused only in 25% of cases. However, there were a few cases of CMS associated with chorioangima. In one report of 13 cases in whom a free-floating intrauterine membrane was found in sonography, one patient with CMS was complicated chorioangioma, but comments or histological specificity of the fluid collection from chorioangioma was not present [11].
In our case, we suspected the etiology of CMS as seromucinous collection from the tumor rather than amnioreduction, because the CMS occurred more than a week after amnioreduction. Furthermore, we were able to observe a large volume of seromucinous material between the amnion and the chorion after uterine incision. Pathological analysis of the mucinous cyst determined that it was a more direct cause than amnioreduction. Mucin-like material secreting chorioangiomas are extremely rare.
Figures and Tables
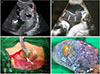 | Fig. 1Antenatal ultrasound and gross findings. (A) 4.84×6.33-cm-sized mixed solid and cystic mass with high vascularity within the placenta (arrow). (B) Extensive subchorionic hypoechogenic lesion (arrow). (C) Operation finding; profuse seromucinous fluid after incision of uterus. (D) Gross appearance of the placental fetal surface; a relatively circumscribed solid mass, measuring up to 6.0 cm in diameter and multiple cystic spaces filled with yellowish fluids. |
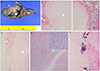 | Fig. 2Pathologic findings. (A) Cut section of placenta shows relatively circumscribed grayish white solid mass (arrow) with focal calcification, measuring 6.0 cm in long diameter, and cystic space (*), filled with seromucinous fluids, and located between placental tissue (p) and mass. (B) Microscopically the cystic spaces (*) between chorioamniotic membrane (m) (H&E, ×40). (C) Placental tissue (p) are filled with fluid materials (H&E, ×40). (D) Another cystic areas between membranous tissues (m) (H&E, ×40). (E) Chorioangioma mass with (F) degenerative change and calcification (H&E, ×40; inset, ×200). |
References
1. Wou K, Chen MF, Mallozzi A, Brown RN, Shrim A. Pregnancy outcomes and ultrasonographic diagnosis in patients with histologically-proven placental chorioangioma. Placenta. 2011; 32:671–674.
2. Sepulveda W, Aviles G, Carstens E, Corral E, Perez N. Prenatal diagnosis of solid placental masses: the value of color flow imaging. Ultrasound Obstet Gynecol. 2000; 16:554–558.
3. Sepulveda W, Alcalde JL, Schnapp C, Bravo M. Perinatal outcome after prenatal diagnosis of placental chorioangioma. Obstet Gynecol. 2003; 102(5 Pt 1):1028–1033.
4. Quintero RA, Reich H, Romero R, Johnson MP, Goncalves L, Evans MI. In utero endoscopic devascularization of a large chorioangioma. Ultrasound Obstet Gynecol. 1996; 8:48–52.
5. Levine D, Callen PW, Pender SG, McArdle CR, Messina L, Shekhar A, et al. Chorioamniotic separation after second-trimester genetic amniocentesis: importance and frequency. Radiology. 1998; 209:175–181.
6. Lewi L, Hanssens M, Spitz B, Deprest J. Complete chorioamniotic membrane separation. Case report and review of the literature. Fetal Diagn Ther. 2004; 19:78–82.
7. Taori K, Patil P, Attarde V, Singh A, Rangankar V. Chorioangioma of placenta: sonographic features. J Clin Ultrasound. 2008; 36:113–115.
8. Amer HZ, Heller DS. Chorangioma and related vascular lesions of the placenta: a review. Fetal Pediatr Pathol. 2010; 29:199–206.
9. Abdalla N, Bachanek M, Trojanowski S, Cendrowski K, Sawicki W. Placental tumor (chorioangioma) as a cause of polyhydramnios: a case report. Int J Womens Health. 2014; 6:955–959.
10. Kim YN, Jeong DH, Jeong SJ, Sung MS, Kang MS, Kim KT. Complete chorioamniotic membrane separation with fetal restrictive dermopathy in two consecutive pregnancies. Prenat Diagn. 2007; 27:352–355.
11. Kaufman AJ, Fleischer AC, Thieme GA, Shah DM, James AE Jr. Separated chorioamnion and elevated chorion: sonographic features and clinical significance. J Ultrasound Med. 1985; 4:119–125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download