Abstract
Objective
To evaluate the complication and recurrence rates in patients undergoing trocar-guided mesh implant for pelvic organ prolapse (POP) treatment.
Methods
A retrospective study was performed based on the medical records of patients who had undergone mesh implant by one surgeon from May 2006 to August 2013 at the Presbyterian Medical Center in Korea. We evaluated perioperative complications such as bladder injury, mesh exposure, urinary symptoms, infections, and chronic pelvic pain. Recurrence was defined as a POP-quantification system stage ≥II or any symptomatic prolapse.
Results
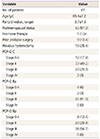
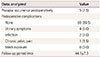
Sixty-seven patients were evaluated, and the mean age of patients was 65.4±7.2 years. Stage ≥III POP-quantification Ba was noted in 61 patients (91%). Intraoperative complications included three cases of bladder injury (4.5%). The mean follow-up period was 44.1±7.9 months. Postoperative complications occurred in seven women (10.5%): four cases of urinary symptoms (6%), two cases of infections (3%), and one case of chronic pelvic pain (1.5%). Mesh exposure did not occur (0%). Prolapse recurrence was reported in five patients (7.5%).
The occurrence of pelvic organ prolapse (POP) in women significantly increases with age [1]. Approximately 40% of women aged 49 to 75 years show evidence of vaginal prolapse, including anterior and vaginal vault prolapse [2]; over 200,000 surgical procedures to remedy this condition are performed in the United States each year [3]. The risk of a POP surgery throughout a woman's life is 11%; 29.2% of these women undergo multiple surgeries [4]. As functional life expectancy increases, the demand for POP repair will also increase [5].
Vaginal extraperitoneal colpopexy with placement of an anterior and posterior mesh using a trocar-based kit is a minimally invasive approach to POP repair [6] that is associated with a good success rate (86.6%) at 1 year postoperatively [7]. However, there is concern that the vaginal mesh implant procedure may present a risk of mesh exposure and poor functional outcomes [8]. The Food and Drug Administration (FDA) has issued safety warnings regarding the use of vaginal mesh implants for POP. Our study aimed to evaluate the complication and recurrence rates in patients undergoing trocar-guided synthetic mesh implant to treat POP.
We retrospectively reviewed medical records (non-randomized) from May 2006 to August 2013 at the Presbyterian Medical Center in Korea. All women presenting with POP were evaluated, including those treated with trocar-guided mesh implants. The medical history included the age, parity, hormonal status, prior prolapse surgery, and physical examination findings were recorded. POP was quantified preoperatively and during follow-up using the POP-quantification (POP-Q) system [9]. The mesh implant surgical technique was standardized according to local protocols [10]. The trocar-guided transvaginal mesh kit, Prolift (Gynecare/Ethicon, West Somerville, NJ, USA), and Easycele system (Biermedics, Wonju, Korea) were used to repair anterior and/or posterior prolapse. Intraoperative characteristics, including the estimated blood loss, type of anesthesia, and operative time, were evaluated. We also noted concomitant hysterectomy as well as intraoperative and postoperative complications. A postoperative gynecological examination was performed at 1 week; 1, 3, 6, and 12 months; and then annually. Failure was defined as recurrent prolapse of stage ≥II or any symptomatic prolapse of the same part at the surgical site.
In addition to the regular physical examination, all patients were contacted by phone in March 2015 to reassess their symptoms and inquire whether any complications occurred or if they required any other treatment related to their initial prolapse surgery. To achieve a longer follow-up period, we did not include patients who had undergone surgery <1 year previously and those who did not respond to the survey. Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). Patients provided informed consent for study participation, and this study was approved by the Presbyterian Medical Center institutional review board.
Sixty-seven women who were treated by vaginal mesh implants to treat POP were evaluated. The median follow-up period was 44.1±7.9 months. The procedures were performed by one gynecologist. Previous hysterectomies had been performed in 19 patients (28.4%). POP-Q Ba of greater than stage III were found in 61 women (91%) (Table 1).
Concomitant vaginal hysterectomy with mesh implants was performed in 34 cases (50.7%). Intraoperative bladder injury occurred in three patients (4.5%) (Table 2), and it occurred in the tenth, forty-seventh, and forty-ninth case of 67 patients. The Prolift system was used in 80.6%, and the Easycele system was used in the remaining patients, since Prolift production was stopped following an FDA safety warning (Table 3).
Postoperative complications occurred in seven cases (10.5%) throughout the follow-up period, which included four cases of urinary symptoms (6%), two cases of infection (3%), and one case of chronic pelvic pain (1.5%). The cases of urinary symptoms included two of incontinence and two of frequency. Mesh exposure did not occur. Urinary symptoms or chronic pelvic pain was considered a complication only when the symptoms were serious in comparison to the symptoms preoperatively or those de novo postoperatively. To classify infection as a complication, it had to have occurred postoperatively and be sufficiently serious enough to require treatment. The prolapse recurrence rate during the follow-up period was 7.5% (Table 4).
The FDA warning published in July 2011 [11] reported the potential risk and complications of mesh implants, prompting a critical debate and exciting developments in POP surgery [12]. After the FDA statement, many vaginal mesh kits for POP repair have been withdrawn from the market; however, comparative studies of vaginal mesh repair and anterior colporrhaphy for anterior compartment prolapse showed a decrease in prolapse recurrence [13]. Recent studies have also reported the anatomical success of transvaginal mesh-augmented reconstruction, demonstrating reinforcement of native connective tissue repair by up to 10% [1415]. In advanced stages of cystocele and recurrent cases, there is often a lack of sufficient connective tissue to achieve reconstruction between the vaginal wall and bladder, leading to an increase of cystocele recurrence of at least 20% and as high as 67% [1617]. Recurrence rates are up to 58% after traditional POP procedures [18]; however, synthetic mesh implants support the weak native connective tissue, which helps fix the prolapsed pelvic compartment, especially the anterior wall. In our study, the postoperatively recurrence rate for POP treatment was 7.5%, which is similar to a previously published recurrence rate of 8.0% after mesh implant [19].
Increased complication rates have also been associated with transvaginal mesh procedures [202122]. In previous studies, mesh exposure was reported as the primary complication contributing to surgical revisions, occurring at rates of 3.2% to 10% [13141923]. Jambusaria et al. [8] noted that the increased risk of complication related to vaginal mesh exposure may be related to the surgical technique, specifically the depth of dissection of the vaginal wall. According to their study, the depth of vaginal incision for mesh placement differs between surgeons. A more superficial dissection causes subsequent superficial placement of the mesh and increased risk of mesh exposure. Marschke et al. [12] reported the surgical technique as one of the main factors for a low erosion rate (3.2%). Procedures performed by experienced surgeons are associated with low complication rates, especially the rate of exposure [24]. In our study, there was no case of mesh exposure or erosion. Moreover, perioperative bladder perforation occurred in three cases (4.5%). The risk of bladder injury related to transvaginal mesh surgery was 1.6% to 3.5% [1325], which was 6.71-fold greater than that of colporrhaphy [26]. The higher frequency of perioperative bladder injury and the lower rate of mesh exposure in our study could be related to the depth of vaginal incision and the surgical technique.
After considering the lower incidence of mesh exposure and a higher rate of bladder perforation compared with previous studies, we conclude that the depth of vaginal incision and dissection affects the risk of complications. The risk of mesh exposure increases when the vaginal incision is more superficial [8]. Conversely, a deep vaginal incision and dissection can increase the risk of bladder perforation. This depends on the surgical technique. Additional studies are needed to determine the relationship between the incision depth and complication rates.
We also evaluated the incidence of urinary symptoms, infection, and chronic pelvic pain after mesh implant. Complications related to surgery occurred in seven women (10.5%). Several studies have reported mesh-related complication rates of 22.5% to 34.8% [2527]. They have been classified as specific mesh-related complications, but these complications, including bladder perforation, were also noted in traditional POP procedures. Therefore, it is difficult to determine whether complications occurring after mesh implant surgery are mesh-related.
In addition, patients with less prominent prolapse (POP-Q <III) repaired by mesh are at a 4-fold greater risk for mesh-related complications [25]. In our study, 76% of patients had undergone anterior repair, and 91% of these had advanced stage POP-Q Ba (POP-Q ≥III). Our focus on advanced-stage patients may explain the lower complication rate in our study compared to that in other studies [252627]. Our findings may support the decision to use appropriate indications for mesh implant surgery.
Different studies have differing conclusions on whether concomitant hysterectomy is a risk factor. According to Kasyan et al. [25], concomitant vaginal hysterectomy leads to a 2.8-fold greater risk of surgical complications and a 2.4-fold greater risk of mesh-related complications. El-Khawand et al. [23] reported that concomitant hysterectomy is an independent risk factor for mesh exposure. They reported that the exposure rate was 23.5% when concomitant hysterectomy was performed compared with 0.8% when hysterectomy was not performed. However, another study showed that previous or concurrent hysterectomy did not affect mesh exposure [28]. A limitation of our study was that 34 cases (50.7%) had concomitant hysterectomy, which makes it difficult to distinguish concomitant surgery as an independent risk factor.
When planning our study, we identified 101 women who underwent mesh surgery by one surgeon during the study period. After excluding patients lost to follow-up, those with a follow-up <1 year, and those who refused to complete the phone survey, 67 cases (66.3%) remained. The retrospective design was the primary limitation of the study, and it is possible that women who had complications and/or recurrences were included among the excluded patients. Thus, our findings may underestimate the number of symptomatic patients.
Since the FDA warning, many reports of complications associated with transvaginal mesh implant surgery for POP have been published. Undoubtedly, tissue support of the mesh implant improves anatomical results and may prevent prolapse recurrences [12]. Conversely, a transvaginal mesh implant is associated with high rates of complications, especially mesh exposure [29], although mesh-related complications are often overestimated or overemphasized, and sometimes poorly expressed [25]. In conclusion, trocar-guided mesh repair for POP had low complication and recurrence rates in our study. Thus, based on our operation results, trocar-guided mesh implant seems to provide safe and effective outcomes. We believe the risk is closely related to factors such as the surgical technique (e.g., the depth of dissection) and stage of POP. Accordingly, we suggest performing trocar-guided mesh repair carefully rather than not performing it at all because of concerns for complications. Therefore, it is important to establish standard management and reporting systems for complications associated with transvaginal mesh graft intervention [30]. The findings of our study may contribute to the development of indications or a manual for surgery.
Figures and Tables
References
1. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002; 31:776–781.
2. Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008; 300:1311–1316.
3. Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007; 369:1027–1038.
4. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997; 89:501–506.
5. Mechanic D. The changing elderly population and future health care needs. J Urban Health. 1999; 76:24–38.
6. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapsed. J Pelvic Med Surg. 2007; 13:351–360.
7. van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008; 199:694.e1–694.e6.
8. Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015; 21:87–92.
9. Persu C, Chapple CR, Cauni V, Gutue S, Geavlete P. Pelvic Organ Prolapse Quantification System (POP-Q): a new era in pelvic prolapse staging. J Med Life. 2011; 4:75–81.
10. Ouzaid I, Hermieu JF, Misraa V, Gosseine PN, Ravery V, Delmas V. Transvaginal repair of genital prolapse using the Prolift technique: a prospective study. Prog Urol. 2010; 20:578–583.
11. US Food and Drug Administration. Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapsed: FDA-Safety Communication [Internet]. Silver Spring (MD): US Food and Drug Administration;2011. cited 2016 Mar 17. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
12. Marschke J, Hengst L, Schwertner-Tiepelmann N, Beilecke K, Tunn R. Transvaginal single-incision mesh reconstruction for recurrent or advanced anterior vaginal wall prolapse. Arch Gynecol Obstet. 2015; 291:1081–1087.
13. Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C. Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med. 2011; 364:1826–1836.
14. Maher CM, Feiner B, Baessler K, Glazener CM. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011; 22:1445–1457.
15. Moore RD, Mitchell GK, Miklos JR. Single-incision vaginal approach to treat cystocele and vault prolapse with an anterior wall mesh anchored apically to the sacrospinous ligaments. Int Urogynecol J. 2012; 23:85–91.
16. Peterson TV, Karp DR, Aguilar VC, Davila GW. Primary versus recurrent prolapse surgery: differences in outcomes. Int Urogynecol J. 2010; 21:483–488.
17. Maher C, Baessler K. Surgical management of anterior vaginal wall prolapse: an evidencebased literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2006; 17:195–201.
18. Miedel A, Tegerstedt G, Morlin B, Hammarstrom M. A 5-year prospective follow-up study of vaginal surgery for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:1593–1601.
19. Kozal S, Ripert T, Bayoud Y, Menard J, Nicolacopoulos I, Bednarzyck L, et al. Morbidity and functional mid-term outcomes using Prolift pelvic floor repair systems. Can Urol Assoc J. 2014; 8:E605–E609.
20. Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010; 17:493–499.
21. Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicenter, randomized, prospective, controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolapse. Am J Obstet Gynecol. 2012; 207:301.e1–301.e7.
22. Vollebregt A, Fischer K, Gietelink D, van der Vaart CH. Primary surgical repair of anterior vaginal prolapse: a randomised trial comparing anatomical and functional outcome between anterior colporrhaphy and trocar-guided transobturator anterior mesh. BJOG. 2011; 118:1518–1527.
23. El-Khawand D, Wehbe SA, O'Hare PG 3rd, Arunachalam D, Vakili B. Risk factors for vaginal mesh exposure after mesh-augmented anterior repair: a retrospective cohort study. Female Pelvic Med Reconstr Surg. 2014; 20:305–309.
24. Murphy M, Holzberg A, van Raalte H, Kohli N, Goldman HB, Lucente V, et al. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication. Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Int Urogynecol J. 2012; 23:5–9.
25. Kasyan G, Abramyan K, Popov AA, Gvozdev M, Pushkar D. Mesh-related and intraoperative complications of pelvic organ prolapse repair. Cent European J Urol. 2014; 67:296–301.
26. Nussler E, Kesmodel US, Lofgren M, Nussler EK. Operation for primary cystocele with anterior colporrhaphy or non-absorbable mesh: patient-reported outcomes. Int Urogynecol J. 2015; 26:359–366.
27. Papcun P, Krizko M Jr, Spodniakova B, Redecha M, Gabor M, Ferianec V, et al. Long term follow-up of the patients with pelvic organ prolapse after the mesh implantation using strict indication criteria. Bratisl Lek Listy. 2014; 115:287–291.
28. Stepanian AA, Miklos JR, Moore RD, Mattox TF. Risk of mesh extrusion and other mesh-related complications after laparoscopic sacral colpopexy with or without concurrent laparoscopic-assisted vaginal hysterectomy: experience of 402 patients. J Minim Invasive Gynecol. 2008; 15:188–196.
29. De Ridder D. Should we use meshes in the management of vaginal prolapse? Curr Opin Urol. 2008; 18:377–382.
30. Firoozi F, Ingber MS, Moore CK, Vasavada SP, Rackley RR, Goldman HB. Purely transvaginal/perineal management of complications from commercial prolapse kits using a new prostheses/grafts complication classification system. J Urol. 2012; 187:1674–1679.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download