Abstract
Objective
The aim of our study is to determine clinical factors and sonographic findings associated with endometrial hyperplasia or cancer (EH+) in premenopausal and perimenopausal women.
Methods
A total of 14,340 transvaginal ultrasonography examinations of 9,888 healthy premenopausal and perimenopausal women were included in this retrospective study. One hundred sixty-two subjects underwent endometrial biopsy based on abnormal uterine bleeding (AUB), sonographic endometrial abnormalities (thickened endometrium, endometrial mass, or endometrial stripe abnormality), or both. The clinical factors and sonographic endometrial abnormalities were evaluated with regard to EH+.
Results
Histologically verified EH+ was found in fourteen subjects (8.6%); ten cases of endometrial hyperplasia (EH) without atypia, three cases of EH with atypia (AEH), and one case of endometrial cancer. Neither clinical factors nor AUB were associated with EH+ (P=0.32) or AEH+ (P=0.72). Of sonographic findings, endometrial stripe abnormality was significantly associated with EH+ (P=0.003) and marginally associated with AEH+ (P=0.05), but a thickened endometrium was not associated with EH+ (P=0.43).
Up to 30% of premenopausal and perimenopausal women experience abnormal uterine bleeding (AUB) during their reproductive years [1], which is often a concern for endometrial hyperplasia or cancer (EH+). In clinical practice, it is often difficult to define AUB in perimenopausal women, who usually present irregular menstruation as their ovarian function declines; in addition, what exactly constitutes the perimenopausal state sometimes differs among clinicians [234]. Although endometrial cancer is primarily a disease of postmenopausal women, up to 14% of those afflicted are premenopausal, among whom 4% are <40 years old [5]. Moreover, due to the Western lifestyle, the increase in obesity and the decline in fertility rate [6], the incidence of endometrial cancer in Korea has steadily increased and become a substantial health concern. As of 2010, 37.1% of cases occurred in women in their 50s, whereas 25.6% of cases occurred in women in their 40s (25.6%), although the incidence is still much lower than in the US or Europe [789].
For these reasons, it could be inferred that endometrial evaluation with transvaginal ultrasonography (TVUS) may have clinical relevance in premenopausal women. That is, this technique may allow for the selection of subjects in whom an early diagnosis of endometrial cancer can be made and who would have the best opportunity to be cured [1011]. Currently, some women often undergo TVUS as part of a routine gynecologic examination, which is commonly performed as a health-checkup. In terms of screening for endometrial cancer by TVUS, investigators have sought to find the optimal cut-off value of endometrial thickness in women with AUB, in particular those with postmenopausal bleeding. Many studies generally define an endometrial thickness of 4.0- or 5.0-mm as the normal cut-off value in postmenopausal women [121314]. Recently, United Kingdom Collaborative Trial of Ovarian Cancer Screening reported that when both endometrial abnormalities and endometrial thickness are considered, TVUS has an increased sensitivity for the detection of endometrial cancer in asymptomatic postmenopausal women [15]. However, this practice has neither been endorsed by professional groups, such as the National Cancer Institute, nor yet established in premenopausal and perimenopausal women [101617].
Therefore, we conducted this study in order to evaluate TVUS findings of the endometrium and clinical factors associated with EH+ in premenopausal and perimenopausal women.
Between August 2010 and June 2013, a total of 26,054 women visited Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea for routine health-checkups. During the check-ups, all participants were asked to complete a standard questionnaire that included demographic characteristics, medical histories, history of abdominal or pelvic surgery, and current medical conditions/medications, including a history of cancer. Information regarding menstrual patterns, reproductive history, history of contraceptive and hormone use, and menopausal status were obtained from a direct interview by three expert gynecologists during TVUS examination. In terms of abnormal menstrual patterns, AUB includes the following: any changes in the menstrual pattern, heavy bleeding (total volume of >80 mL), frequent (interval between the onset of bleeding episodes is less than 21 days) or prolonged menstruation (longer than seven days), and bleeding/spotting at an abnormal time (intermenstrual bleeding). Prolonged menstrual bleeding, even in perimenopausal women, was also considered to be AUB.
Based on the responses to the questionnaires and medical interviews, we excluded subjects with all types of known invasive gynecologic cancers or those with any personal history of hysterectomy and/or unilateral/bilateral salpingo-oophorectomy. Women who had any procedures or who had taken any medications for the treatment of endometrial neoplasia were also excluded from this study. We also excluded women of postmenopausal status, which was defined as the absence of menses for at least 1 year or a level of serum follicle-stimulating hormone of 40 IU/L or higher; therefore, women who had missed two or more cycles of menstruation or who experienced an interval of amenorrhea of more than 60 days within 12 months were considered perimenopausal, unless serum follicle-stimulating hormone levels or pregnancy indicated otherwise.
For the evaluation of the uterus and bilateral adnexae, TVUS with GE LOGIQ 9 (GE Healthcare, Chalfont St. Giles, UK) ultrasound machines was performed by three gynecologists with more than eight years of experience, who were diplomats of the gynecologic specialty. Representative gray-scale images and measurements of each ovary and the uterus were archived at the picture archiving communication system at our center. During the TVUS, endometrial thickness was measured at its thickest point from the anterior to the posterior in the sagittal plane of the uterus. Calipers were placed perpendicularly to the outer edge of the endometrium. If there was fluid in the endometrial cavity, the endometrial thickness was measured as described above, but with the inclusion of the endometrial cavity fluid and double endometrial lining; then, the fluid diameter was subtracted at the same point. In addition to measurement of the endometrium, any other details of the endometrium were recorded; whether the endometrium was regular, cystic, heterogeneous or abnormally distended and had a polypoid, a mass or any other type of lesion were all included under the definition of sonographic endometrial abnormalities [18].
For the purpose of this investigation, we categorized sonographic endometrial abnormalities as a thickened endometrium, an endometrial mass and an endometrial stripe abnormality. First, a thickened endometrium was defined as follows: thickness was dependent on the menstrual cycle and varied between the proliferative phase (4 to 8 mm) and the secretory phase (8 to 14 mm) in premenopausal women; the 8-mm cutoff value was used for perimenopausal women unless they presented with other AUB [1920]. Thus, with regard to endometrial thickness, we distinguished normal endometrium from thickened endometrium. Second, apart from endometrial thickness, the presence of an endometrial mass and/or an endometrial stripe abnormality was noted for each subject. Irrespective of endometrial thickness, a single or two discrete and well-demarcated polyp-like endometrial lesion was categorized as an endometrial mass. With the exception of endometrial mass, any irregular, cystic or heterogeneous multiple polypoid masses, an abnormally distended endometrium, or fluid in the endometrial cavity were included within the definition of endometrial stripe abnormalities. Not all so-nographic endometrial abnormalities were criteria for immediate intervention by endometrial biopsy. Only women with sonographic endometrial abnormalities who experienced a recent episode of AUB or asymptomatic women who presented with a definitely thickened endometrium of 20-mm or greater were asked to undergo an immediate endometrial biopsy. For asymptomatic subjects, follow-up TVUS was performed at the end of menstruation, or 3 months later in the case of perimenopausal women with prolonged amenorrhea. During follow-up, endometrial biopsy was also recommended for subjects who still demonstrated a thickened endometrium of 8-mm or more, who experienced AUB at any time, or who demonstrated persistent or aggravated endometrial stripe abnormalities [15]. A small, asymptomatic endometrial mass (<10 mm) alone was not recommended for endometrial biopsy unless its size had increased by the time of the follow-up TVUS.
Dilatation and curettage was performed at the Gangnam center day surgery clinic, under local anesthesia with midazolam/demerol and/or with a paracervical block containing 20 mL of 2% lidocaine for intracervical infiltration. The cervix was dilated up to Hegar-10, if possible. First, any polypoid masses were removed, and then endometrial tissue was obtained by curettage; assistance or guidance by transvaginal/transabdominal ultrasonography was used when necessary. Biopsy specimens of polypoid masses and endometrial tissues were fixed separately in formaldehyde (neutral buffered formalin), embedded in paraffin, and stained with hematoxylin and eosin. The histologic diagnosis was given according to the World Health Organization criteria.
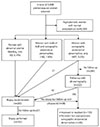
Among the women who were screened, we reviewed 9,888 premenopausal and perimenopausal women (43.0±7.0 years old) who underwent 14,340 health-checkups that included TVUS for screening of the pelvic cavity as part of a gynecologic evaluation. Finally, the eligible study population consisted of subjects who underwent endometrial biopsy for their AUB or sonographic endometrial abnormalities (Fig. 1). Written informed consent was obtained from all participants. This retrospective cross-sectional study was approved by the institutional review board of Seoul National University Hospital (H-1210-120-437) and was performed in accordance with the ethical standards described in the Declaration of Helsinki.
The primary analysis of this study focused on the clinical or sonographic factors associated with simple/complex EH with and without atypia or cancer, due to the very low prevalence of atypical EH or cancer in the study population.
To investigate the relationship between epidemiological/clinical variables and endometrial lesions, we categorized the following variables: body mass index (BMI; <23 kg/m2, normal vs. ≥23 kg/m2, overweight) [21], parity (0 vs. 1 or more), sonographic endometrial abnormalities (for endometrial thickness, normal vs. thickened endometrium; for endometrial mass, absent vs. present; for endometrial stripe abnormality, absent vs. present) and/or AUB (absent vs. present). With respect to tumor markers, serum levels of CA-125 and CA-19-9 were each measured on the same day as the TVUS examination, with a cut-off value of 37.0 mIU/L. The baseline characteristics of continuous variables were compared using the Student's t-test or Mann-Whitney U-test. For categorical variables, a chi-square test or Fisher exact test was used. Data were analyzed with IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA), and P-values of <0.05 were considered statistically significant after a two-tailed test.
Among the 9,888 premenopausal and perimenopausal women (43.0±7.0 years old) from the health-checkups, AUB was reported in 4.6%, and the percentage of women with significant AUB that prompted an endometrial biopsy was 2.0%. Sonographic endometrial abnormalities were noted in 6.7% of women who were screened; a thickened endometrium (2.3%), suspected endometrial masses (4.6%), and endometrial stripe abnormalities (0.8%), or two or more (1.0%). A total of 223 women were initially asked to undergo an endometrial biopsy because of their AUB (n=40), sonographic endometrial abnormalities (n=21), or both (n=162). Of the 476 asymptomatic women with sonographic endometrial abnormalities, 42 subjects who had developed AUB or had aggravated abnormal TVUS findings of the endometrium were recommended for endometrial biopsy. In total, an endometrial biopsy was suggested for 265 women. A total of 162 women with available histologic data comprised our study population (Fig. 1).
The mean age of the 162 eligible subjects was 44.9±5.1 years (range, 28 to 54 years), and the average BMI was 21.5 kg/m2; a normal BMI (<23.0 kg/m2) was defined in 71.3% of the participants. The mean parity was 1.6±0.8 (range, 0 to 3).
The final pathologic diagnosis following endometrial biopsy by dilatation and curettage was in 14/162 (8.6%) with EH+; ten cases of EH without atypia, three cases of endometrial hyperplasia with atypia (AEH) and one case of endometrial cancer. Of the benign pathologies, the presence of endometrial polyps (77/162, 47.5%) was the most common finding after a normal endometrium (63/162, 38.9%). There were no procedure-related complications.
The association of each clinical factor and sonographic finding with EH+ is shown in Table 1. After analysis of the clinical factors, no significant associations of nulliparity and overweight (BMI ≥23 kg/m2), hypertension, history of cancer or history of endometrial biopsy with EH+ were observed. Only 1.52% of our study population used oral contraceptives, and therefore, we could not provide further analysis on the association of the birth control pill with EH+. Serum tumor markers did not exhibit any significant differences among women with and without EH+. The presence of AUB was not significantly associated with either EH+ (P=0.32) or AEH+ (P=0.72).
As an integrated value, sonographic endometrial abnormalities are not related to EH+ (P=0.88) or AEH+ (P=0.47). Of the sonographic endometrial abnormalities, however, endometrial stripe abnormality alone was identified as having a significant association with EH+ (P=0.003), but were only marginally associated with AEH+ (P=0.05). On the other hand, focal endometrial mass, such as suspicious endometrial polyp, or thickened endometrium had no association with either EH+ (P=0.12 for endometrial mass, P=0.43 for thickened endometrium, respectively) or AEH+ (P=0.63 for endometrial mass, P=0.15 for thickened endometrium, respectively, data not shown).
AUB is the cardinal symptom of endometrial cancer [22], and is often a concern for EH+. Although most women with endometrial cancer have a high survival rate [23], still, stage for stage, survival rates for endometrial cancer are similar to ovarian cancer. With respect to screening for endometrial cancer, the finding of asymptomatic endometrial thickening discovered during TVUS often raises a physician's concern. The optimal cut-off of endometrial thickness in women without AUB has been to be practically defined by TVUS, which would allow clinicians to ascertain any potential malignancies or premalignancies of the endometrium [24], but has not yet been established. Nevertheless, the TVUS still remain the mainstay of the gynecologic examination and therefore it seems to be inferred that endometrial evaluation with TVUS may have a clinical role in screening for endometrial cancer, as asymptomatic women with endometrial cancer have a higher rate of well-differentiated tumors compared with women with postmenopausal bleeding prior to diagnosis [25].
Our study showed that neither AUB nor thickened endometrium were significantly related with EH+. According to our study protocol, in which subjects who had a definite, thickened endometrium during their menstrual cycle were requested to undergo an endometrial biopsy irrespective of the presence of symptoms, endometrial thickness was relatively thicker in asymptomatic women compared with symptomatic women (14.4±4.4 vs. 13.0±5.2 mm, respectively P=0.08, data not shown). Endometrial thickness was not significantly different among women with and without EH+ (13.5±5.2 vs. 13.4±5.0 mm, P=0.92, data not shown). When only women with AUB were considered, there was no difference in endometrial thickness between women with and without EH+ (13.4±4.7 vs. 12.9±5.3 mm, P=0.75, data not shown). Among the women who had a thickened endometrium as defined by the study protocol or that was more than 20-mm, there were none with AEH+ based on the biopsy. By contrast, endometrial stripe abnormalities were found to be significantly associated with EH+ in premenopausal and perimenopausal women with/without AUB. Therefore, the thickened endometrium itself may not provide a perspective in the context of EH+, while endometrial stripe abnormalities, such as heterogeneity or cystic changes to the endometrium, may be crucial in the delineation of EH+ even in asymptomatic premenopausal and perimenopausal women.
Another concern is whether the screening of endometrial cancer by TVUS is clinically relevant in asymptomatic premenopausal and perimenopausal women with no complaints of symptoms, such as AUB. Many women are often unaware of whether their menstrual patterns are abnormal [26], although AUB is one of the most common symptoms that cause women to seek medical attention [1]. In addition, the presentation of AUB depends upon each woman's subjective experience and on the impression of the amount of blood loss; therefore, less than half of the women who seek medical attention for menstrual complaints meet the criterion of AUB, which is defined as greater than 80 mL of menstrual blood loss per cycle [27]. In some studies, a majority of women who complained of AUB presented within a normal range of menstrual variation, whereas up to 17% of women who attend routine evaluations and do not express complaints about their menstruation were screened and found to have experienced AUB [2628]. Even practitioners use confusing and inconsistent definitions to refer to abnormal menstrual bleeding [29].
Of all the premenopausal and perimenopausal women who visited our institution for their health check-ups, 4.6% reported AUB, but through gynecologic interview, only 1.4% of cases were defined as significant AUB that required an endometrial biopsy. Interestingly, all clinical factors, including AUB, were not associated with EH+ or AEH+. Among the four subjects who had AEH+, a 49-year-old woman who had presented with prolonged menstruation that persisted for 7 days and was perceived as perimenopausal state revealed an 11.7 mm endometrial thickness with minute cystic changes on TVUS. She was finally diagnosed with AEH by dilatation and curettage. Another 43-year-old premenopausal woman with persistent menorrhagia had experienced severely intractable iron deficiency anemia, even though endometrial polyps were removed 2 years before this. In this case, TVUS led to the discovery of endometrial stripe abnormality and irregularly shaped hyperechogenic masses in the absence of thickened endometrium for menstrual cycle days; subsequent explorative laparotomy confirmed a well-differentiated endometrioid adenocarcinoma. Consequently, we could infer that as long as accompanied by interviews performed by gynecologists, an endometrial evaluation by TVUS might be useful in the determination of endometrial premalignancies or malignancies of healthy premenopausal and perimenopausal women with no complaints of AUB.
One unavoidable limitation of this study is that because our institution is a healthcare system with a focus on screening as part of a routine health-checkup program, endometrial biopsies by dilation, curettage, and biopsy could not be performed in all consecutive subjects asked, due to either endometrial abnormalities found during TVUS or due to significant AUB. In addition, the TVUS protocol used in this study was based on the practice of the Gangnam center, which is, for the most part, compatible with the current practice within Korea; however, the final decision rested with each clinician. A more specific limitation is that eligible subjects of our study comprised a very small number of cases of EH+ (14/162, 8.6%), including one case of endometrial cancer and three cases of AEH. Accordingly, the primary target included cases of simple or complex EH, although atypical EH is considered pre-malignancies of the endometrium [3031]. Nevertheless, this is unlikely to alter the performance of TVUS with respect to observation of the endometrium.
Our results show that endometrial stripe abnormality observed by TVUS is a significant risk factor associated with EH+ in premenopausal and perimenopausal women with and without AUB. To the best of our knowledge, this is the largest epidemiologic study of endometrial evaluation by TVUS in healthy premenopausaldl and perimenopausal women of a wide age-range that represents the reproductive population of Korea.
In conclusion, endometrial stripe abnormality other than endometrial thickness as measured by TVUS is considered to be significant finding in the recommendation of endometrial biopsy to exclude EH+ even in asymptomatic premenopausal and perimenopausal women. In view of the rising incidence of endometrial cancer, provided a systematic, protocol-driven characterization of endometrial abnormalities (i.e., endometrial stripe abnormalities), such as the International Ovarian Tumor Analysis, a morphology index based on a scoring system for ovarian tumors [3233], TVUS would be a helpful screening tool to exclude endometrial premalignancies or malignancies even in premenopausal or perimenopausal women who do not experience AUB that would require medical attention.
Figures and Tables
References
1. Brenner PF. Differential diagnosis of abnormal uterine bleeding. Am J Obstet Gynecol. 1996; 175(3 Pt 2):766–769.
2. Goldstein SR, Zeltser I, Horan CK, Snyder JR, Schwartz LB. Ultrasonography-based triage for perimenopausal patients with abnormal uterine bleeding. Am J Obstet Gynecol. 1997; 177:102–108.
3. Astrup K, Olivarius Nde F, Moller S, Gottschau A, Karlslund W. Menstrual bleeding patterns in pre- and perimenopausal women: a population-based prospective diary study. Acta Obstet Gynecol Scand. 2004; 83:197–202.
4. Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss: a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966; 45:320–351.
5. Ash SJ, Farrell SA, Flowerdew G. Endometrial biopsy in DUB. J Reprod Med. 1996; 41:892–896.
6. Bray F, Dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005; 14:1132–1142.
7. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009; 24:995–1003.
8. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.
9. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
10. Dreisler E, Sorensen SS, Ibsen PH, Lose G. Value of endometrial thickness measurement for diagnosing focal intrauterine pathology in women without abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2009; 33:344–348.
11. Dubinsky TJ. Value of sonography in the diagnosis of abnormal vaginal bleeding. J Clin Ultrasound. 2004; 32:348–353.
12. Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand. 2002; 81:799–816.
13. Getpook C, Wattanakumtornkul S. Endometrial thickness screening in premenopausal women with abnormal uterine bleeding. J Obstet Gynaecol Res. 2006; 32:588–592.
14. Karlsson B, Granberg S, Wikland M, Ylostalo P, Torvid K, Marsal K, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding: a Nordic multicenter study. Am J Obstet Gynecol. 1995; 172:1488–1494.
15. Jacobs I, Gentry-Maharaj A, Burnell M, Manchanda R, Singh N, Sharma A, et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011; 12:38–48.
16. Smith-Bindman R, Kerlikowske K, Feldstein VA, Subak L, Scheidler J, Segal M, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998; 280:1510–1517.
17. Breitkopf DM, Frederickson RA, Snyder RR. Detection of benign endometrial masses by endometrial stripe measurement in premenopausal women. Obstet Gynecol. 2004; 104:120–125.
18. Bredella MA, Feldstein VA, Filly RA, Goldstein RB, Callen PW, Genant HK. Measurement of endometrial thickness at US in multicenter drug trials: value of central quality assurance reading. Radiology. 2000; 217:516–520.
19. Carey WD. Cleveland Clinic Foundation. Current clinical medicine. Philadelphia: Saunders Elsevier;2010.
20. Smith RP. Association of Professors of Gynecology and Obstetrics. Clinical management of abnormal uterine bleeding. Crofton (MD): Association of Professors of Gynecology and Obstetrics;2006.
21. World Health Organization Regional Office for the Western Pacific Region. International Association for the Study of Obesity. International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment [Internet]. Manila: World Health Organization Regional Office for the Western Pacific Region;2000. cited 2016 Mar 17. Available from: http://www.wpro.who.int/nutrition/documents/Redefining_obesity/en.
22. Kimura T, Kamiura S, Yamamoto T, Seino-Noda H, Ohira H, Saji F. Abnormal uterine bleeding and prognosis of endometrial cancer. Int J Gynaecol Obstet. 2004; 85:145–150.
23. National Cancer Institute. SEER stat fact sheets: endometrial cancer [Internet]. Bethesda (MD): National Cancer Institute;cited 2014 Jun 20. Available from: http://seer.cancer.gov/statfacts/html/corp.html.
24. Ozdemir S, Celik C, Gezginc K, Kiresi D, Esen H. Evaluation of endometrial thickness with transvaginal ultrasonography and histopathology in premenopausal women with abnormal vaginal bleeding. Arch Gynecol Obstet. 2010; 282:395–399.
25. Seebacher V, Schmid M, Polterauer S, Hefler-Frischmuth K, Leipold H, Concin N, et al. The presence of postmenopausal bleeding as prognostic parameter in patients with endometrial cancer: a retrospective multi-center study. BMC Cancer. 2009; 9:460.
26. Geller SE, Harlow SD, Bernstein SJ. Differences in menstrual bleeding characteristics, functional status, and attitudes toward menstruation in three groups of women. J Womens Health Gend Based Med. 1999; 8:533–540.
27. Warner PE, Critchley HO, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol. 2004; 190:1216–1223.
28. Geller SE, Bernstein SJ, Harlow SD. The decision-making process for the treatment of abnormal uterine bleeding. J Womens Health. 1997; 6:559–567.
29. Fraser IS, Critchley HO, Munro MG. Abnormal uterine bleeding: getting our terminology straight. Curr Opin Obstet Gynecol. 2007; 19:591–595.
30. Torres ML, Weaver AL, Kumar S, Uccella S, Famuyide AO, Cliby WA, et al. Risk factors for developing endometrial cancer after benign endometrial sampling. Obstet Gynecol. 2012; 120:998–1004.
31. Maxwell GL, Schildkraut JM, Calingaert B, Risinger JI, Dainty L, Marchbanks PA, et al. Progestin and estrogen potency of combination oral contraceptives and endometrial cancer risk. Gynecol Oncol. 2006; 103:535–540.
32. Ueland FR, DePriest PD, Pavlik EJ, Kryscio RJ, van Nagell JR. Preoperative differentiation of malignant from benign ovarian tumors: the efficacy of morphology indexing and Doppler flow sonography. Gynecol Oncol. 2003; 91:46–50.
33. Kaijser J, Bourne T, Valentin L, Sayasneh A, Van Holsbeke C, Vergote I, et al. Improving strategies for diagnosing ovarian cancer: a summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound Obstet Gynecol. 2013; 41:9–20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download