Abstract
Microcystic stromal tumor (MCST) of the ovary is a rare subtype of ovarian tumor first described in 2009. Although high nuclear expression of β-catenin and β-catenin gene (CTNNB1) mutation are related with ovarian MCST, the origin and genetic background of ovarian MCST remain unclear. In this study, two cases of ovarian MCST are presented. Microscopically, the tumors showed a microcystic pattern and regions with lobulated cellular masses with intervening hyalinized, fibrous stroma. Tumor cells of both cases were stained with CD10, vimentin, and Wilms tumor 1. Genetic analysis was performed and β-catenin gene (CTNNB1) mutation in exon 3 was detected in both cases. This is the first report in regards of detecting CTNNB1 mutation in ovarian MCST through the use of pyrosequencing (a novel sequencing technique).
Microcystic stromal tumor (MCST) of the ovary is a rare subtype of ovarian tumor with less than 30 cases reported worldwide [123456]. It is a new disease entity first described by Irving and Young in 2009 [1] based on 16 cases of uncategorized ovarian tumors. The distinctive histological characteristics of the tumor include: a microcystic pattern and regions with lobulated cellular masses with intervening, sometimes hyalinized, fibrous stroma; an absence of morphologic features enabling any other specific diagnosis in the sex cord-stromal category; an absence of epithelial elements; and an absence of teratomatous or other germ cell elements. Immunohistochemically, MCST cells are strongly positive for CD10, vimentin, and Wilms tumor 1, and negative for sex-cord stromal markers (α-inhibin and calretinin) and epithelial membrane antigen.
The origin and genetic background of ovarian MCST is still unclear. In 2011, Maeda et al. [2] reported β-catenin gene (CTNNB1) mutation and high nuclear expression of β-catenin in two cases of ovarian MCST. Several more reports that mutation in β-catenin gene (CTNNB1) is related with ovarian MCST followed [3456]. In this study, we report two cases of ovarian MCST, along with the β-catenin gene (CTNNB1) mutation in exon 3 that was also detected in both cases. This is the first report of the detection of CTNNB1 mutation in ovarian MCST using pyrosequencing.
A 24-year-old female, gravida 0, visited the outpatient department because of lower abdominal discomfort for a week. After physical evaluation, the patient underwent computed tomography (CT), which showed an 18×12-cm multilocular cystic mass originating from the left adnexa. The cyst presented features of benign epithelial ovarian cyst such as smooth outer wall and thin internal sepation (Fig. 1A). Serum levels of CA-125 and CA-19-9 were within normal limits. The patient underwent laparoscopic salpingo-oopherecomy. Due to material insufficiency, the result of intraoperative frozen biopsy was non-diagnostic. Finally, the patient was diagnosed as MCST. No adjuvant treatment was performed. The patient is currently free of disease after 8 months from surgery.
A 31-year-old woman, gravida 0, who presented with a palpable mass at lower abdomen was referred to a tertiary healthcare institution. Pelvis CT and pelvis magnetic resonance imaging revealed a 24-cm-sized left ovarian mass with solid and cystic portion. The mass presented thick outer wall with irregular margin (Fig. 1B, C). For further examination, positron emission tomography (PET)-CT was performed. On PET-CT, intense 18-fluoro-2-doxy-D-glucose uptake was shown at 24-cm-sized left ovarian mass and several lymph nodes in aortocaval space and left paraaortic space, which suggested ovarian malignancy with lymph node metastasis (Fig. 1D-F). Elevation of tumor markers reflected ovarian epithelial tumor. CA-125 was elevated at 205.3 IU/L (normal range, 0 to 35 IU/L). Human epididymis protein 4 was slightly elevated at 69.1 IU/L (normal range, 0 to 67 IU/L). The patient underwent staging laparotomy including left salpingo-oopherecomy, paraaortic lymph node sampling, and abdominal peritonectomy. The result of intraoperative frozen pathology was "favoring sex cord stromal tumor". After the immunohistochemical examination, the patient was finally diagnosed as MCST and paraaortic lymph nodes were free from tumor. Without adjuvant therapy, she is currently free of disease after 3 months after surgery and scheduled for follow-up after 6 months.
Grossly, the resected specimen of case 1 was fragmented tumor tissues (18×12 cm), consisting of a relatively well-circumscribed cystic mass, necrotic material, and blood clots (Fig. 2A). The cystic wall containing tumor tissue showed a smooth outer surface and internal thin, fibrous septae. No definite solid lesion was identified. The specimen of case 2 was a well-circumscribed, multilobulated cystic mass measuring 20×14 cm and weighing 80 g (Fig. 2B). Extensive tumor necrosis was identified within the mass. Multifocal solid lesions were identified. There was no capsular involvement.
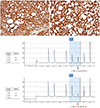
Microscopically, the tumor consisted of three histologic components: solid sheets of tumor cells, numerous microcysts, and intervening bundles of hyalinized fibrous bands within the stroma (Fig. 2C, D). The variable-sized microcysts were lined with bland-appearing tumor cells (Fig. 2E, F). The individual tumor cells had uniform, small, round nuclei with inconspicuous nucleoli and abundant granular, eosinophilic cytoplasm. Immunohistochemically, the tumor cells exhibited cytoplasmic immunoreactivity for CD10, CD99, and vimentin (Fig. 3A), as well as diffuse and strong nuclear immunoreactivity for β-catenin (Fig. 3B).
In addition, pyrosequencing revealed a point mutation (S33C) in exon 3 of CTNNB1 (Fig. 3C, D), confirming the diagnosis of ovarian MCST.
MCST of the ovary is a newly categorized ovarian tumor first described by Irving and Young in 2009 [1] based on 16 cases of uncategorized ovarian tumors. Although MCST is sometimes mistaken for the coma due to its thick hyalinized fibrous band, it has distinctive microscopic features of solid, microcystic or macrocystic patterns, and uniform and round tumor cells with low number of mitotic figures [47]. Behavior of the tumor is not well known because of limited follow-ups. The available information would suggest it is likely benign in most cases [1].
As the name hints, the origin of ovarian MCST is thought to be stroma. Irving and Young suggested possibility of stromal origin by excluding other origins. MCST's characteristic immunoprofile (CD10-positive, vimentin-positive, and epithelial membrane antigen-negative) and ultrastructural analysis also supports the possibility of stromal origin. However, Maeda et al. [2] insisted MCST should be classified as "tumors of uncertain origin" considering the pluripotency of the tumor cells.
In 2011, Maeda et al. [2] reported β-catenin gene (CTNNB1) mutation and high nuclear expression of β-catenin in two cases of ovarian MCST. Since 2011, eleven more MCST cases were reported, with β-catenin gene (CTNNB1) mutation confirmed in eight cases [3456]. β-catenin is a regulatory protein in cell to cell adhesion and gene transcription [8]. Dysregulation of β-catenin signaling is known to be associated with malignancies, such as colon cancer, hepatocellular carcinoma, and endometrial cancer [39]. Nature of the β-catenin protein and frequent expression rate of β-catenin gene (CTNNB1) mutation in MCST suggests β-catenin plays an important role in pathogenesis of the tumor.
However, there are few cases of non-β-catenin gene (CTNNB1) mutation MCST. Recently, Lee et al. reported a case of ovarian MCST in a familial adenomatous polyposis patient. In this case, the β-catenin gene (CTNNB1) mutation was not detected. Instead, a somatic mutation of the APC gene in exon 11, with a heterozygous deletion at nucleotide position c.1540delG was discovered. Yang and Bhattacharjee [3] reported a case of ovarian MCST with aberrant accumulation of nuclear p27 protein. Because p27Kip1 is regarded as tumor suppressor gene [10], Yang and Bhattacharjee [3] described the possibility that dysregulation of p27Kip1 plays a role in development of MSCT. However, there have been no further studies about the relationship between accumulation of p27 protein and MCST. Also in this study, genetic analysis was performed for only CTNNB1 mutation which is known as the key gene alteration of MSCT.
One more thing to emphasize is that pyrosequencing was conducted for gene analysis. Pyrosequencing is a novel method of DNA sequencing based on the sequencing by synthesis. It takes less time and little effort analyzing genetic alteration, compared to conventional Sanger sequencing. This result will help deciding methods for genetic analysis in future MCST studies.
In conclusion, we report two cases of ovarian MCST with its unique histologic and immunohistochemical characters. Through the use of pyrosequencing, a point mutation in β-catenin gene in exon3 was confirmed. As far as we know, β-catenin gene mutation is the key gene alteration of MSCT. Ovarian MCST is known as benign ovarian tumor, and further studies of its genetic background, treatment, and prognosis are needed.
Figures and Tables
Fig. 1
(A) Computed tomography (CT) findings of case 1. An 18×12-cm-sized cystic mass with smooth outer wall with no definite solid portion, suggesting benign epithelial ovarian tumor. (B) CT findings of case 2 presents a 24-cm-sized solid and cystic left ovarian mass, suspicious for malignant epithelial tumor. No pathologically enlarged lymph node or seeding mass was observed in the imaging study. (C) Magnetic resonance imaging findings of case 2 suggests malignant epithelial tumor of ovary. (D-F) Positron emission tomography-CT findings of case 2 shows intense uptake by a 24-cm-sized left ovarian mass and several lymph nodes in aortocaval space and left paraaortic space, which suggests ovarian malignancy with lymph node metastasis.
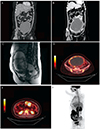
Fig. 2
Gross (A,B) and microscopic (C-F) findings. (A) Case 1. Fragmented specimen shows an admixture of gray-to-yellow, viable tumor tissues and red-to-tan, necrotic and hemorrhagic tissues. (B) Case 2. The resected specimen reveals a cystic mass displaying extensive internal tumor necrosis and hemorrhage. The cystic wall has multiple areas of irregular, nodular thickening, indicating viable tumor tissues. (C) Low-power view of case 1. Small, irregular, occasionally coalescing cystic spaces are lined by bland cells (H&E, ×100). (D) Low-power view of case 2. An area showing solid component exhibits similar nuclear features (H&E, ×40). (E,F) High-power views of (E) case 1 (H&E, ×400) and (F) case 2 demonstrate uniform, small, round to ovoid nuclei with absent or inconspicuous nucleoli. No mitotic activity is detected (H&E, ×200).
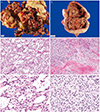
Fig. 3
Immunohistochemical findings (A,B) and pyrosequencing results (C,D). Immunostaining reveals the tumor cells are positive for (A) vimentin (immunohistochemistry; HRP-multimer method, ×200). (B) In addition, diffuse and strong nuclear immunoreactivity for β-catenin was identified, suggesting the nuclear translocation and accumulation of β-catenin protein in the individual tumor cells (immunohistochemistry; HRP-multimer method, ×400). (C,D) Pyrograms of a DNA sequence featuring a point mutation involving a single base pair substitution (c.98C>G; pS33C) in exon 3 of CTNNB1 gene. The mutated site is highlighted in blue and the allele frequencies are given in each table. (C) Case 1. (D) Case 2.
Acknowledgments
This study was supported by grants from the National Research Foundation of Korea Grant funded by the Korean Government (2011-0010800 and 2014-002926).
References
1. Irving JA, Young RH. Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm. Am J Surg Pathol. 2009; 33:367–375.
2. Maeda D, Shibahara J, Sakuma T, Isobe M, Teshima S, Mori M, et al. β-catenin (CTNNB1) S33C mutation in ovarian microcystic stromal tumors. Am J Surg Pathol. 2011; 35:1429–1440.
3. Yang M, Bhattacharjee MB. Ovarian microcystic stromal tumor: report of a new entity with immunohistochemical and ultrastructural studies. Ultrastruct Pathol. 2014; 38:261–267.
4. Kang YN, Cho CH, Kwon SY. Microcystic stromal tumor of the ovary with mutation in exon 3 of β-catenin: a case report. Int J Gynecol Pathol. 2015; 34:121–125.
5. Lee SH, Koh YW, Roh HJ, Cha HJ, Kwon YS. Ovarian microcystic stromal tumor: a novel extracolonic tumor in familial adenomatous polyposis. Genes Chromosomes Cancer. 2015; 54:353–360.
6. Bi R, Bai QM, Yang F, Wu LJ, Cheng YF, Shen XX, et al. Microcystic stromal tumour of the ovary: frequent mutations of β-catenin (CTNNB1) in six cases. Histopathology. 2015; 67:872–879.
7. Reichert RA. Diagnostic gynecologic and obstetric pathology. 1st ed. Philadelphia (PA): Lippincott Williams & Wilkins;2012.
8. Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999; 21:1021–1030.
9. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17:9–26.
10. Berton S, Belletti B, Wolf K, Canzonieri V, Lovat F, Vecchione A, et al. The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition. Mol Cell Biol. 2009; 29:5031–5045.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download