Abstract
Endometrial cancer is the third most common gynecologic cancer in the Korea and occurs mainly in menopausal women. Although it can develop in young premenopausal women cancer as well, an attack in the adolescent girl is very rare. A 13-year-old girl visited gynecology department with the complaint of abnormal uterine bleeding. An endometrial biopsy revealed FIGO (International Federation of Gynecology and Obstetrics) grade II endometrial adenocarcinoma. In the treatment of endometrial cancer, conservative management should be considered if the patient is nulliparous or wants the fertility preservation. Therefore, we decided to perform a hormonal therapy and a follow-up endometrial biopsy after progestin administration for eight months revealed no residual tumor. We report a case of endometrial cancer occurred in a 13-year-old girl with a brief review of the literature.
Endometrial cancer is the third most common gynecologic cancer in the Korea following cervical and ovarian cancers. However, the incidence of endometrial cancer has been increasing during 1999–2010 with an annual percent changes of 6.9%, and shows a distinctly increasing pattern in younger and older females (annual percent changes in females <30 and >80 years old were 11.2% and 9.5%, respectively) [1]. Endometrial cancer occurs mainly in menopausal women, but it can develop in younger women as well, 2% to 14% of cases occur in women with 40 years old or younger [23]. Endometrial cancer occurred in adolescents is extremely rare, but the youngest patient reported in literature was 14-year-old girl [45].
The standard treatment of endometrial cancer consists of a total hysterectomy, bilateral salpingo-oophorectomy and/or pelvic lymphadenectomy. These options can be applied to a menopausal or a premenopausal women who do not care for the conception. However, conservative therapeutic options should be considered for the young women who want to preserve their fertility. Although the medical treatment with progestogens could be a reasonable option to young patients with grade I, stage IA endometrial cancer under the complete evaluation and careful selection, all the risks should be informed to the patients because this is not a standard therapy.
We present a case of endometrial cancer occurred in 13-year-old girl, the youngest case in our knowledge, with a brief review of the literature. This report was an exempt from deliberation of our institutional review board (Keimyung University Dongsan Medical Center).
A 13-year-old virginal girl visited gynecology department with a complaint of abnormal uterine bleeding for one year. Menarche was at 10-year-old and she had suffered from irregular menstruation and heavy menstrual bleeding since the menarche. She had severe anemia, hemoglobin level was 5.7 g/dL, which was corrected to 9.0 g/dL after transfusion of four units of packed red blood cells. Her height and weight were 160.7 cm and 63.5 kg, and resulting body mass index was 24.8 kg/m2 (in Asian women; normal range, 18.5 to 23 kg/m2) [6]. She had no remarkable medical or family history except appendectomy. A trans-rectal ultrasonography showed thickened endometrium of 18 mm with focal microcystic appearance and polycystic ovaries. Magnetic resonance imaging revealed a focal nodular mass involving the endometrial mucosa of the fundus (Fig. 1) and no demonstrable myometrial invasion. Lymph nodes were not involved. Under the impression of endometrial pathology, endometrial biopsy was performed with regional anesthesia. A histologic examination confirmed the endometrial adenocarcinoma arising in atypical complex hyperplasia that was well differentiated and grade II according to the FIGO (International Federation of Gynecology and Obstetrics) 2000 classification of endometrial cancer (Fig. 2A, B) [7].
Under the diagnosis of stage IA, grade II endometrial adenocarcinoma, megestrol acetate (megestrol acetate suspension; Daewon Pharmaceuticals, Seoul, Korea) was administrated with a dose of 160 mg daily for 3 months. A follow-up endometrial biopsy after the medication showed asynchronous endometrium with multifocal degenerated atypical glands (Fig. 2C). Treatment was continued with medroxyprogesterone acetate (Provera, Pfizer Pharmaceuticals, New York, NY, USA) with a maintenance dose of 10 mg daily for 5 months, the second and third follow-up endometrial biopsy revealed asynchronous endometrium due to hormonal effect with no residual tumor (Fig. 2D-F). Follow-up magnetic resonance imaging also showed no endometrial mass or hyperplasia. It has taken 8 months after medications till a complete remission. The patient made a gain of 14 kg in weight for the 8 months of medications. The weight gain was an only side effect of medical treatment and a source of anxiety, but it was tolerable without any interventions.
The patient is currently in good health with levonorgestrel-releasing intrauterine device (Mirena, Bayer Korea Pharmaceuticals, Seoul, Korea).
The known risk factors for developing endometrial hyperplasia or cancer are obesity, nulliparity, infertility, hypertension and diabetes, and all these factors are also known to be associated with polycystic ovary syndrome [8]. Our patient also could be diagnosed as polycystic ovary syndrome. Most risk factors are related with prolonged, unopposed estrogen stimulation. However, there are two pathogenetic types in an endometrial cancer. Type I is estrogen-dependent, and occurs in younger women, begin as endometrial hyperplasia and progress to carcinoma. The other hand, type II endometrial cancer is estrogen-independent, and not associated with endometrial hyperplasia, and tend to occur in older, postmenopausal women. Also, type I tumors tend to be well differentiated and have a more favorable prognosis compared with type II tumors [9].
Among the risk factors for endometrial cancer, inherited factors have also been considered as important risk factors, especially in young women with endometrial carcinoma. Hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome is characterized by the development of endometrial and colon cancers. This syndrome arise from defects in the genes responsible for repair of mismatched DNA; such as mutations in the DNA mismatch repair genes, MLH1, MSH2, MSH6, PMS1, and PMS2 [10]. It was reported that 11.2% of Korean women affected in the endometrial cancer met the criteria for clinical or suspected-HNPCC [10]. Therefore, genetic screening or a direct genetic study should be considered in a younger patients or a patients with family history for HNPCC. In our case, we could not find any hereditary relations from a detailed family history, so, did not perform a genetic study.
Fertility preservation is the most important issue for the young girl, so conservative therapeutic options should be considered. Fortunately, conservative management can be considered for young women because of their good prognosis as mentioned before. Hormone therapy alone or combined with hysteroscopic ablation are known as the most useful and effective conservative management [11]. A conservative treatment for fertility preservation can be applied to the patients with early endometrial cancer (stage I, grade I). Hormone therapy comprises mainly progestogens, which is classified into oral and intrauterine device. Hormonal treatment is usually indicated in the case of well differentiated, stage I endometrial cancer, especially in the case with positive progesterone receptors, and the absence of lymphovascular invasion and accompanied ovarian tumor. Medroxyprogesterone acetate (400 to 600 mg/day) and megestrol acetate (160 to 200 mg/day) were usually used for oral treatment [811]. The response rate has been reported as 57% to 75% and the recurrence rate ranges from 11% to 50%. As an alternative option to oral route, levonorgestrel-releasing intrauterine device (IUD) can be used. It could cure the some cases of stage IA, grade I disease combined with oral medroxyprogesterone or not [2]. The progesterone therapy combined with hysteroscopic ablation has been proposed as a new therapeutic model. Twenty patients with endometrial cancer, grade I, stage IA with positive estrogen and progesterone receptor underwent hysteroscopic ablation of the tumor and received hormone therapy, megestrol acetate (160 mg/day) or levonorgestrel (52 mg) IUD for 6 months. All but one responded to that treatment [1213].
After completion of their family plan, hysterectomy should be proposed as a final treatment. But, Bovicelli et al. [11] insisted that hysterectomy was not required for all women because of the low risk of recurrence (35%).
The majority of the literatures relevant to the conservative management dealt with endometrial cancer grade I, stage IA. Brown et al. [14] reported a case of an adolescent girl with grade II endometrioid adenocarcinoma cured with levonorgestrel IUD. But, Mitamura et al. [5] reported a 14-year-old girl with a grade II endometrial cancer treated with medroxyprogesterone acetate, who didn't respond to treatment and underwent hysterectomy. In Mitamura's case, hormonal agent was prescribed only for one month, and it is presumed that the duration of medication was too short to cure the disease. In our case, initially, oral progestogen was administered for 8 months, and followed by LNG-IUS therapy, and complete remission was attained at 8 months after treatment.
In postmenopausal women, endometrial thickness is positively correlated with the presence of endometrial disease and endometrial biopsy can be decided according to the endometrial thickness. However, in premenopausal women, there are no clinical guideline to determine when the endometrial biopsy should be performed. Of the many clinical factors, endometrial thickness has been known as the most important factor to predict the endometrial hyperplasia or cancer. Although endometrial cancer in adolescence is very rare and there is difficulty in examination due to their virginity, the physician should consider the endometrial biopsy to rule out endometrial cancer if there are clinical findings such as thickened endometrium, intermenstrual interval of more than 3 months or clinical history suggesting long-term unopposed estrogen exposure.
Figures and Tables
Fig. 1
Magnetic resonance imaging showing nodular mass involving the fundus and diffuse superficial infiltration along the entire posterior wall.
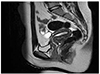
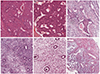
Fig. 2
Histopathologic findings of the consecutive endometrial biopsies (H&E, ×110). First biopsy shows adenocarcinoma (A) arising in the atypical complex hyperplasia (B). (C) Second biopsy shows asynchronous endometrium with only focal atypical glands and degenerated morular lesions. (D) Third biopsy shows only asynchronous endometrium with atrophic glands. Fourth biopsy shows asynchronous endometrium (E) and pseudodecidualization (F).
References
1. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013; 24:298–302.
2. Gerli S, Spano F, Di Renzo GC. Endometrial carcinoma in women 40 year old or younger: a case report and literature review. Eur Rev Med Pharmacol Sci. 2014; 18:1973–1978.
3. Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001; 167:39–48.
4. Lee CM, Szabo A, Shrieve DC, Macdonald OK, Gaffney DK. Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma. JAMA. 2006; 295:389–397.
5. Mitamura T, Watari H, Todo Y, Koshida T, Sakuragi N. A 14-year-old female patient with FIGO stage IB endometrial carcinoma: a case report. Int J Gynecol Cancer. 2009; 19:896–897.
6. Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002; 360:235.
7. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000; 70:209–262.
8. Fadhlaoui A, Ben Hassouna J, Khrouf M, Zhioua F, Chaker A. Endometrial adenocarcinoma in a 27-year-old woman. Clin Med Insights Case Rep. 2010; 3:31–39.
9. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983; 15:10–17.
10. Lim MC, Seo SS, Kang S, Seong MW, Lee BY, Park SY. Hereditary non-polyposis colorectal cancer/Lynch syndrome in Korean patients with endometrial cancer. Jpn J Clin Oncol. 2010; 40:1121–1127.
11. Bovicelli A, D'Andrilli G, Giordano A, De Iaco P. Conservative treatment of early endometrial cancer. J Cell Physiol. 2013; 228:1154–1158.
12. Mazzon I, Corrado G, Masciullo V, Morricone D, Ferrandina G, Scambia G. Conservative surgical management of stage IA endometrial carcinoma for fertility preservation. Fertil Steril. 2010; 93:1286–1289.
13. Laurelli G, Di Vagno G, Scaffa C, Losito S, Del Giudice M, Greggi S. Conservative treatment of early endometrial cancer: preliminary results of a pilot study. Gynecol Oncol. 2011; 120:43–46.
14. Brown AJ, Westin SN, Broaddus RR, Schmeler K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet Gynecol. 2012; 119(2 Pt 2):423–426.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download