Abstract
Spontaneous complete chorioamniotic membrane separation (CMS) without invasive fetal procedure is extremely rare and associated with adverse perinatal outcomes. A woman with complete CMS which was detected at the 21 weeks' gestation. She did not take any fetal invasive procedures before the diagnosis. At 27 weeks' gestation, an emergency Caesarean section was performed because of fetal distress. The defect of the uterine muscle was detected on the fundus. The baby has grown well without any morbidity. This is the first reported case of complete CMS relative to uterine scar. And we suggest that the pregnancy can be maintained successfully if there is no fetal abnormality when complete CMS is detected on ultrasound.
Complete chorioamniotic membrane separation (CMS) occurs extremely rarely without invasive fetal procedure. CMS has been associated with adverse perinatal outcomes [1]. We present the case that CMS occurred spontaneously without invasive fetal procedures or fetal structural abnormality. We infer that the uterine scar detected during Cesarean section was one of the possible reasons for the CMS, and that the uterine muscle defect had been caused by the previous dilatation and curettage before this pregnancy.
A healthy 35-year-old woman, gravida 3 para 1, was referred to our unit at 21 weeks and 5 days' gestation because of CMS. The course of the pregnancy had been uneventful until 16 weeks with no history of amniocentesis or abdominal trauma. The patient had two previous pregnancies. The first pregnancy had been uneventful, resulting in a caesarean delivery at term. The second pregnancy was terminated by dilatation and curettage because of a missed abortion at 8 weeks'
gestation.
An ultrasound examination revealed a single living fetus with an estimated weight of 419 g. However, the amniotic membrane appeared to be in a completely separated state from the chorion except the site where the umbilical cord was inserted into the placenta. The amount of amniotic fluid was decreased overall in the amniotic cavity, while the echogenicity of the amniotic fluid was increased. In contrast, the fluid between the separated amnion and chorion had low echogenicity (Fig. 1A, B). We tried to perform a targeted ultrasound examination for fetal anomaly; however, it was difficult to scan the fetal structure in detail due to oligohydramnios.
After obtaining the patient's consent, we removed 20 mL of amniotic fluid for chromosome analysis and cytology and then slowly infused 230 mL of warm saline and 40 mg of Indigocarmine (United Pharm, Seoul, Korea) into the amniotic cavity with a 22-gauge spinal needle. After infusion, we could confirm the fetus had no evidence of fetal anomaly, including in its urinary system. About ten minutes later, the amniotic fluid in the amniotic cavity started to decrease to the previous size. In contrast, the space between the chorion and the amnion had enlarged. After removal of 200 mL of amniotic fluid from the space, we completed the procedure. There was no evidence of leakage of amniotic fluid into the vagina. Based on this outcome, our diagnosis was CMS with amniotic membrane rupture. Chromosome analysis revealed 46XX and cytology showed there was no no white blood cell in the amniotic fluid and its glucose level was 24 mg/dL.
At 26 weeks and 6 days' gestation, she complained of regular uterine contractions. We used betamethasone for fetal lung maturation and magnesium sulfate as tocolytics. At 27 weeks and 4 days' gestation, fetal electric cardiac monitor showed minimal variability of heart rate and recurrent variable deceleration. An emergency caesarean section was performed. The newborn was female and weighed 930 g without gross anomaly. Apgar scores at 1 and 5 minutes were 4 and 6. She was intubated and transferred to the neonatal intensive care unit.
After the delivery of the baby and placenta, a localized mushy surface measuring 1.5 cm in diameter was found on the fundus of the uterus. There was no evidence of placenta accrete. We put one hand through the uterine incision and guessed at the thickness of the squashy uterine wall with the other hand over the uterine surface. There was the defect of the uterine muscle and the thickness of the remnant wall was less than 5 mm (Fig. 1C). We could infer that the defect was one of the convincing reasons for the CMS, and that the uterine muscle defect had been caused by the previous dilatation and curettage before this pregnancy. There was complete CMS on the placenta and amniotic membrane was only attached where the umbilical cord was inserted on the placental disc (Fig. 1D); pathology revealed acute chorioaminonitis, deciduitis, and acute funisitis. After delivery, maternal body temperature decreased to a normal range. The patient was discharged without any complications. Although the baby was diagnosed with respiratory distress syndrome and treated in the neonatal intensive care unit, she was discharged without complications 14 weeks after birth. The baby's current age is 15 months, and she has grown well without any morbidity.
CMS is a detachment between the chorion and the amnion. It can be diagnosed after 16 weeks of gestation because chorion-amnion fusion is normally completed within 16 weeks [1]. Although there is no formal grading system for CMS, the degrees of separation can be identified according to severity. Partial CMS is a partial separation at one site and complete CMS is entire detachment except the site where the umbilical cord was inserted into the placenta [2]. CMS is very rare. The incidence of CMS detected by prenatal ultrasonography has been reported as ranging from 1:187 to 1:4333 [23]. Excluding partial CMS, the incidence of complete CMS is expected to be much lower.
Previous studies report that CMS can happen for two reasons. The first reason is a lack of chorion-amnion fusion, which usually occurs between 14 and 16 weeks of gestation. Incomplete chorion-amnion fusion is generally associated with aneuploidy (e.g., trisomy 21, 13, and 18) or fetal connective tissue disorders (e.g., restrictive dermopathy) [145]. The second reason for CMS is considered iatrogenic complication caused by invasive fetal procedures such as amniocentesis, cordocentesis, fetoscopy or fetal surgery [367]. However, spontaneous, complete CMS without invasive procedure or trauma is extremely rare and an exact cause has not yet been found.
In this case, CMS occurred spontaneously without invasive fetal procedures. Furthermore, the fetus did not have any structural abnormality. Up to now, English-language medical reports have no cases with a healthy baby born without aneuploidy and structural abnormalities who was affected by spontaneous complete CMS except one case with CMS associated with extremely short umbilical cord [8]. Meanwhile, it is noteworthy that the maternal uterus had a muscle defect on the fundus. There seems to be a high probability that the uterine muscle defect in this case caused CMS by reducing the tension of the chorion and weakening the bonding force between the chorion and amnion. Therefore, we suggest that uterine scar formed before the pregnancy must be considered as one of the risk factors for spontaneous CMS.
Membrane separation can lead to preterm premature rupture of the membranes and preterm labor by releasing chemical mediators. In addition, the disruption of membranes can make the protective layers of the amniotic cavity more sensitive to infection and cause chorioamnionitis. Actually, CMS has been associated with adverse perinatal outcomes such as preterm delivery, fetal growth restriction, and fetal death in previous reports [27]. Here as elsewhere, the pregnancy was to end in a preterm birth at 27 weeks and 4 days' gestation due to preterm premature rupture of the membranes and chorioamnionitis.
However, in this report, the baby is growing well without any physical or mental morbidity until now. Here, we suggest that the pregnancy can be maintained if there is no evidence of fetal abnormality when complete CMS is found on the ultrasound. If spontaneous CMS is detected in the pregnancy that has no history of invasive fetal procedures, chromosome analysis and detailed ultrasound examination are needed to determine normal fetus, because CAS is rarely isolated and is more often associated with fetal aneuploidy [4]. After the age of viability, careful examination must be performed to evaluate fetal well-being. Serial ultrasound examinations are needed to assess fetal growth, amniotic fluid volume, the presence of amniotic band formation, and presence of possible compression of the umbilical cord. The biophysical profile score must be determined by ultrasonography and fetal electrocardiography. Doppler exams which include the umbilical artery, middle cerebral artery, and ductus venosus can be helpful [9]. Some reports have mentioned that hospitalization for close monitoring is necessary to reduce fetal morbidities and mortality [29]. This case is very significant in two regards. Firstly, this shows that uterine scar formed before the pregnancy can be one of the risk factors for spontaneous CMS, and secondly, a healthy baby was safely delivered and has grown well without morbidity until 15 months after birth even though complete CMS. Although CMS may be a serious prenatal condition, a good outcome can be obtained.
Figures and Tables
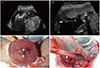 | Fig. 1Complete chorioamniotic membrane separation. (A) Ultrasonographic image at 21 weeks' gestation, showing the amnion (arrowhead) separated from the chorion (arrow) and fetus in amniotic cavity. (B) Ultrasonographic image at 21 weeks' gestation, showing normal-looking placenta and decreased amount of amniotic fluid volume in the amniotic cavity; chorion (arrow), amnion (arrowhead). (C) A localized mushy surface (arrow) measuring 1.5 cm in diameter was found on the fundus of the uterus, where was the defect of the uterine muscle. (D) Placenta reveals the amnion (arrowhead) separated from the chorion (arrow). |
References
1. Kim YN, Jeong DH, Jeong SJ, Sung MS, Kang MS, Kim KT. Complete chorioamniotic membrane separation with fetal restrictive dermopathy in two consecutive pregnancies. Prenat Diagn. 2007; 27:352–355.
2. Sydorak RM, Hirose S, Sandberg PL, Filly RA, Harrison MR, Farmer DL, et al. Chorioamniotic membrane separation following fetal surgery. J Perinatol. 2002; 22:407–410.
3. Egawa M, Hayashi S, Yang L, Sakamoto N, Sago H. Chorioamniotic membrane separation after fetoscopic laser surgery for twin-twin transfusion syndrome. Prenat Diagn. 2013; 33:89–94.
4. Abboud P, Mansour G, Zejli A, Gondry J. Chorioamniotic separation after 14 weeks' gestation associated with trisomy 21. Ultrasound Obstet Gynecol. 2003; 22:94–95.
5. Bronshtein M, Zimmer EZ. Oligohydramnios with amniochorionic separation at 15-16 weeks' gestation. Prenat Diagn. 1995; 15:161–164.
6. Lewi L, Hanssens M, Spitz B, Deprest J. Complete chorioamniotic membrane separation. Case report and review of the literature. Fetal Diagn Ther. 2004; 19:78–82.
7. Bromley B, Shipp TD, Benacerraf BR. Amnion-chorion separation after 17 weeks' gestation. Obstet Gynecol. 1999; 94:1024–1026.
8. Kasuga Y, Miyakoshi K, Ikenoue S, Kadohira I, Matsumoto T, Minegishi K, et al. Complete chorion-amnion separation presenting as a stuck fetus. Acta Obstet Gynecol Scand. 2013; 92:990–991.
9. Ishikawa G, Satomi M, Inagawa-Ichikawa T, Abe T, Akira S, Takeshita T. A case report of complete chorioamniotic membrane separation. J Nippon Med Sch. 2011; 78:120–125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download