Abstract
Aggressive angiomyxoma, a rare soft tissue benign neoplasm, predominantly occurs in the female pelvic peritoneum and perineum region during reproductive age. It is slow growing, locally infiltrative, and has a high risk of local recurrence and the neoplastic character of blood vessels. The standard treatment is surgery. We report three unusual aggressive angiomyxoma cases. The first case was a pedunculated mass of the left labium major; the second, a left perineal mass that infiltrated into the paravesical area via the obturator foramen; and the third, a big mass in the retroperitoneal cavity, found that growing aggressive angiomyxoma looked like lava expulsion in the pelvic area. After a thorough examination and full radiologic workup, we performed surgical excision in each patient via different approaches. Histopathologic findings were consistent with diagnosis of aggressive angiomyxoma. To date, no relapse has been observed.
Aggressive angiomyxoma (AAM), a rare soft tissue neoplasm, predominantly occurs in the female pelvic peritoneum and perineum region. These lesions are distinguished by their slow growth and frequent recurrences, and are characterized histologically by a predominantly myxoid stroma and abundant thin- and thick-walled vascular channels. Because of its rarity, AAM is often initially misdiagnosed as a gynecological malignancy. We also found that AAM looked like lava expulsion in the pelvic cavity. After a thorough examination and full radiologic workup, we performed surgical excision, using a different surgical approach for each growth site.
A 49-year-old woman presented with a large vulvar mass. She previously had a much smaller vulvar mass for a year, and had been asymptomatic. The size of the mass suddenly increased in a month. Examination revealed a large polypoid pedunculated mass arising from the left labium major, with ulceration and secondary infection (Fig. 1A-a, b). There were no palpable masses upon examination of the abdominal and groin areas. Pelvic computed tomography revealed a bulging mass (19×19 cm), with mild non-homogeneous enhancement along the ventral margin of the pelvic wall and major labium. No pelvic mass was observed; there was no free fluid or hydronephrosis. The mass was excised using a 1-cm lateral margin, with the patient under general anesthesia. No recurrence has been observed 4 years after excision.
On pathological analysis, an aggressive vulvar angiomyxoma, 27×24 cm in size and 1,520 g in weight was found (Fig. 1A-c, d). Immunohistochemical staining showed positivity for estrogen receptor, CD24, desmin, and S100. The patient has been regularly followed-up for 4 years and is not undergoing adjuvant therapy.
A 31-year-old woman complained of progressive abdominal distension and lower abdominal swelling. Examination revealed a distended, slightly tender abdomen. Ultrasonography revealed a huge, mixed echogenic mass that looked like fluid collection in the whole pelvic cavity. We suspected hemoperitoneum. Computed tomography (CT) scanning showed an 18×15×8-cm high-density cystic mass and fluid collection in the pelvis with diffuse peritoneal thickening (Fig. 2A). The patient had a normal menstrual history, no operation history, and had never been diagnosed with hepatitis or tuberculosis. Blood serum tests were negative for hepatitis B and C viruses.
Laparoscopic surgery was performed under general anesthesia. However, after finding the huge solid mass, we converted to laparotomy to excise the tumor. The tumor extended from the para-aortic bifurcation to the paravesical and pararectal spaces in the retroperitoneal area (Fig. 1B-a-c). We dissected the mass while preserving the uterus, ovaries, and rectosigmoid colon. Intra-operative frozen section diagnosis was that of a mesenchymal tumor. Specimen histopathology showed capillaries and cavernous vascular spaces filled with blood, and stellate spindle cell proliferation in interstitial tissue (Fig. 2C). Immunohistochemical staining showed positivity for CD10, smooth muscle actin, desmin, and CD34. These findings were consistent with AAM diagnosis. The patient has had no recurrence 12 months after following surgery, and has been regularly followed-up.
A 36-year-old woman presented with a progressive perineal mass on the left buttock. She was performed excisional biopsy due to suspicious fibroma. Physical examination revealed swelling and a dense lesion on the left buttock (Fig. 1C-a-d). Rectal examination revealed that the mass was not related to the rectum. Magnetic resonance imaging (MRI) showed a 15×10×6-cm irregularly shaped enhanced mass in the left perineum, extending to the left retroperitoneum via the obturator foramen (Fig. 2B). In the lithotomy position with general anesthesia, the tumor was excised via the perineal approach (Fig. 1C-c), and confirmed remaining mass via abdominal laparotomy. Histopathology of the specimen also showed capillaries and cavernous vascular spaces filled with blood, and stellate spindle cell proliferation in the interstitial tissue. Immunohistochemical staining showed positivity for CD10, smooth muscle actin, desmin, and CD34. The patient has had no recurrences 4 months after surgery and has been regularly followed-up.
AAM is a slow-growing mesenchymal neoplasm with a high local recurrence rate, but with a low tendency to metastasis. It was first described as a distinct variant of myxoid neoplasms in 1983 [1]. These tumors mostly occurs in the 4th decade of life and 95% of them occur in women [2]. It typically presents as a vulvar polypoid mass, and diagnosed using histology. The tumors are sometimes positive for estrogen and progesterone receptors [1]. They are thus likely to grow during pregnancy and respond to hormonal management [3]. AAM occurs mainly in the pelvis, perineum, vulva, vagina, and urinary bladder, and may exert pressure on adjacent organs. Appropriate management and long-term follow-up of this tumor should be considered owing to its locally aggressive nature [4]. This tumor also involves the blood vessels. It can affect the vulva [5] and other parts of the pelvis [6]. This disease has locally infiltrative and recurrent characters. It presents as a painless gelatinous mass and can simulate Bartholin gland cyst or inguinal hernia-like symptoms. Many options have been used for treating recurrences, with varying success, and no single modality is clearly more beneficial than others [1]. Although it is a benign tumor and does not invade the neighboring tissue, it has a tendency to recur after surgical excision [2]. Recurrence can occur at same site after the initial resection [7]. The incidence of local recurrence after surgery is 36% to 72%. The recurrence rate in patients with narrow surgical margins is not higher than in patients with wide surgical margins. The reported age at presentation is 6 to 77 years, with peak occurring incidence during the reproductive years. The female-to-male ratio is 6.6/1. AAM should be distinguished from benign tumors with a low risk of local recurrence as well as from malignant tumors with widespread metastatic potential.
AAM has a characteristic appearance on CT and MRI, and these techniques reveal the tumor extent. On CT, the tumor has a well-defined margin and attenuation less than that of muscles. On T2-weighted MRI, the tumor has high signal intensity [8]. After diagnosis, preoperative angiographic embolization and preoperative external beam irradiation can be helpful to decrease local recurrence [9].
On gross examination, these tumors are typically soft, bulky masses [7]. The external surface is smooth and usually appears not to be encapsulated. Some have projections resembling fingers that extend into neighboring tissues. The cut surface reveals a grey tumor of homogeneous consistency with focal areas of congestion and hemorrhage. Histologically, AAM are mesenchymal tumors, composed of fibroblasts within a strong myxoid background. The tumor consists of scattered hypo-cellular and fusiform cells with thin cytoplasmic processes in a loose myxoid matrix that gives the tumor a pale-pink color on eosin staining. The tumor has also a prominent vascular component with distinct smooth muscle cells without anastomosis. Mitosis is usually not observed, and occasional cases may show mild atypia. Immunohistochemical staining of the tumor for desmin, vimentin, CD34, CD44, S100, smooth-muscle actin, and muscle-specific actin are necessary for the diagnosis [71011]. The term AAM was chosen to emphasize the neoplastic nature of the blood vessels, its locally infiltrative nature and the high risk of local recurrence, but not to indicate its malignant nature [7]. The pathogenesis of AAM is poorly understood. Non-random involvement of the 12q15 region, where the high mobility group protein HMGA2 (an architectural transcription factor expressed primarily during embryogenesis) is located, has been suggested [10]. AAM resection is sometimes difficult because of its infiltrative nature, and recurrence occurs in 70% of cases, but even patients with clear resection margins can develop recurrence [3]. Nonetheless, excision with wide tumor-free margins is still the most common treatment [3]. Incomplete resection is acceptable when high operative morbidity is anticipated or preservation of fertility is an issue [57]. Possible alternative treatment methods for recurrences are gonadotrophin-releasing hormone (GnRH) agonist or antihormonal therapy (tamoxifen) [711]. Several beneficial results have been described using a GnRH agonist [12]. However, long-term GnRH agonist therapy is associated with adverse effects such as menopausal symptoms and bone loss. Potential disadvantages are that the tumor will become resistant to GnRH agonist therapy, and medication withdrawal may result in neoplasm regrowth [7121314]. The optimal duration of therapy is not known, but long term GnRH agonist therapy is not recommendable because of its potential adverse effects [12]. Chemotherapy and radiation therapy are not considered owing to low mitotic activity of the tumor [7]. For estrogen receptor and/or progesterone receptor positive tumors, hormone treatment is a good option. Long-term follow-up and careful monitoring with imaging techniques are essential for timely identification of recurrence [10]. In conclusion, AAM is a rare mesenchymal benign tumor that occurs primarily in the vulvar, vaginal, perineal, and pelvic soft tissues in women of reproductive age. The tumor grows slowly but infiltrates into the softer pelvic spaces in a way similar to lava expulsion from a volcano. Awareness of this disease and full radiologic workup is necessary for surgical planning and recurrence monitoring.
Figures and Tables
Fig. 1
Aggressive angiomyxoma: gross finding. (A) Case 1. A huge perineal mass that reveals aggressive angiomyxoma. (A-a,b) A huge polypoid pedunculated mass that measures 27 cm, with an attached stalk and overlying skin on the left labium is observed. (A-c,d) The mass shows a glistening surface and gelatinous cut surface. (B) Case 2. The retroperitoneal aggressive growth looks like lava expulsion. (B-a) The image shows the pelvic brim. (B-b) The image shows the left adnexal area and posterior cul-de-sac. (B-c) The image shows the protruding mass in the right pelvic retroperitoneum. (B-d) The image shows the excised tumor. (C) Case 3. The tumor lesion extended from the left paravesical space and obturator space, retroperitoneum to the left buttock. (C-a,b) External showing of mass on left labium major to left buttock. (C-c,d) Mass removed from retroperitoneal space which extended to paravesical and obtulator space, over 20-cm-sized irregular shaped.
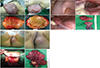
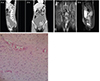
Fig. 2
Radiologic image and microscopic finding. (A) A computed tomography scan in case 2 shows an 18×15×8-cm large, high-density cystic mass, suspected to be fluid collection in the pelvis, with diffuse peritoneal thickening. (A-a) Sagittal view and (A-b) coronal view. (B) This magnetic resonance imaging scan in case 3 shows a 15×10×6-cm large, irregularly shaped enhanced mass in the left perineum, extending to left retroperitoneum via the obturator foramen. (B-a) Coronal view of pelvis on T2 weighted image. (B-b) Sagittal view on T2 weighted image, distinguished with bladder, uterus, and rectum. (C) This image shows the microscopic photograph of the aggressive angiomyxoma (H&E stain, ×200).
References
1. Kura MM, Jindal SR, Khemani UN. Aggressive angiomyxoma of the vulva: an uncommon entity. Indian Dermatol Online J. 2012; 3:128–130.
2. Dahiya K, Jain S, Duhan N, Nanda S, Kundu P. Aggressive angiomyxoma of vulva and vagina: a series of three cases and review of literature. Arch Gynecol Obstet. 2011; 283:1145–1148.
3. Sereda D, Sauthier P, Hadjeres R, Funaro D. Aggressive angiomyxoma of the vulva: a case report and review of the literature. J Low Genit Tract Dis. 2009; 13:46–50.
4. Amin A, El Badawy S, Bull A. Aggressive angiomyxoma of the vulva. J Obstet Gynaecol. 2013; 33:325–326.
5. Mandal S, Dhingra K, Roy S, Khurana N. Aggressive angiomyxoma of the vulva presenting as a pedunculated swelling. Indian J Pathol Microbiol. 2008; 51:259–260.
6. Mathieson A, Chandrakanth S, Yousef G, Wadden P. Aggressive angiomyxoma of the pelvis: a case report. Can J Surg. 2007; 50:228–229.
7. Dierickx I, Deraedt K, Poppe W, Verguts J. Aggressive angiomyxoma of the vulva: a case report and review of literature. Arch Gynecol Obstet. 2008; 277:483–487.
8. Outwater EK, Marchetto BE, Wagner BJ, Siegelman ES. Aggressive angiomyxoma: findings on CT and MR imaging. AJR Am J Roentgenol. 1999; 172:435–438.
9. Nyam DC, Pemberton JH. Large aggressive angiomyxoma of the perineum and pelvis: an alternative approach. Report of a case. Dis Colon Rectum. 1998; 41:514–516.
10. Nucci MR, Fletcher CD. Vulvovaginal soft tissue tumours: update and review. Histopathology. 2000; 36:97–108.
11. Basak S, Rogers S, Solomonsz AF. Superficial angiomyxoma of the vulva: a case report of a rare cutaneous tumour. J Obstet Gynaecol. 2011; 31:360–361.
12. Sun NX, Li W. Aggressive angiomyxoma of the vulva: case report and literature review. J Int Med Res. 2010; 38:1547–1552.
13. McCluggage WG, Jamieson T, Dobbs SP, Grey A. Aggressive angiomyxoma of the vulva: dramatic response to gonadotropin-releasing hormone agonist therapy. Gynecol Oncol. 2006; 100:623–625.
14. Shinohara N, Nonomura K, Ishikawa S, Seki H, Koyanagi T. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int J Urol. 2004; 11:432–435.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download