Abstract
Congenital afibrinogenemia/hypofibrinogenemia is a rare inherited hematologic disorder in which a patient lacks or has insufficient level of fibrinogen, the blood coagulation factor I. The incidence of this uncommon disease is 1 to 2 per 1 million individuals. Hence, massive hemoperitoneum caused by ovulation in a woman with congenital afibrogenemia is also a very rare clinical condition. Massive hemoperitoneum usually presents as acute abdominal pain with potential findings of peritonitis including abdominal distention, hypotension and tachycardia with critical consequences. We performed emergent endoscopic surgery for hemoperitoneum caused by a ruptured corpus luteum cyst in a patient with congenital hypofibrinogenemia. To the best of our knowledge, this was the first case report of such treatment in Korea.
Spontaneous and idiopathic hemoperitoneum refers to intraperitoneal bleeding with or without trauma history. Delayed treatment due to misdiagnosis caused by ambiguous clinical signs may result in serious complications and high mortality rates. Although heavy bleeding in the abdominal cavity due to ovulation is rare, it can be life-threatening to women with coagulopathy and requires surgical treatment.
Congenital afibrinogenemia/hypofibrinogenemia as a hereditary autosomal recessive hematologic disease characterized by complete lack or insufficiency of fibrinogen, the blood coagulation factor I [1]. In two thirds of patients with congenital hypofibrinogenemia, bleeding tendency occurs from infancy with a mild to severe degree and frequency of bleeding [23]. Reports of intra-abdominal bleeding in women with primarily ruptured corpus luteal cyst and hemoperitoneum are extremely rare. We reported a novel case of an 18-year-old female patient with congenital hypofibrinogenemia who developed intra-abdominal hemorrhage due to a ruptured corpus luteum cyst.
A 18-year-old nulliparous woman presented to the department of obstetrics and gynecology with severe abdominal pain and dizziness. Her last menstrual period occurred 15 days prior and her past menstrual pattern was regular. She was diagnosed with congenital hypofibrinogenemia in childhood. Family history indicated that her mother and grandmother had been diagnosed as congenital hypofibrinogenemia. Before the patient visited our department, she had exhibited no bleeding episode except for childhood epistasis. General examination revealed marked pallor, tachycardia and hypotension (pulse 120 bpm, blood pressure 90/60 mmHg). On examination, her abdomen was tender and tense. A urine pregnancy test was negative.
Transvaginal ultrasonography was suggestive of massive hemoperitoneum and a right complex adnexal mass of 6×4×4 cm. Laboratory findings were as follows: hemoglobin level 10.3 g/dL, hematocrit 30.5%, platelets 205,000×109/L. The coagulation profile revealed a prothrombin time of 25/12 seconds (patient/control), activated partial thromboplastin time of 29.3/30 seconds (patient/control), and plasma fibrinogen level of 15 mg/dL (normal 160 to 350 mg/dL). Liver function tests were normal.
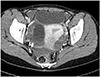
Abdominal computed tomography scan revealed intra-abdominal bleeding due to the rupture of a corpus luteum cyst with massive hematoma (Fig. 1).
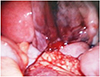
Within 2 hour of hospitalization, the hemoglobin level had decreased to 7.4 g/dL. Three units of red blood cell suspension and 6 units of fresh frozen plasma were transfused immediately. However, exploratory laparoscopy was performed since the hemodynamic parameters remained unstable and the abdominal distention worsened. Intraoperatively, approximately 2.5 L of blood was evacuated. We diagnosed a ruptured ovulatory follicle on the right ovary (Fig. 2). The main bleeding site was partially excised and cauterized, preserving the ovary. Intraoperatively, 3 more units of red blood cell suspension and 10 units of fresh frozen plasma were transfused. The postoperative period was uneventful and the patient was advised to take prolonged low-dose oral contraceptive pills.
The total absence or insufficiency of fibrinogen is caused by mutation in 1 of 3 genes i.e., fibrinogen alpha-chain (FGA), beta-chain (FGB), and gamma-chain (FGG) on chromosome 4q [1]. It is a very rare disease with the incidence of 1-2 in 1 million individuals. Approximately 250 cases have been reported with equivalent incidence in males and females [23].
In general, bleeding may be easily detected if hematoma is developing in skin tissue during situations of mucosal damage like tooth brushing, simple bumping, vomiting or even delivery. Mild bleeding, as compared to more severe bleeding in hemophilia patients is common and the patient may remain asymptomatic for a long time. Other symptoms such as severe bleeding due to minor trauma, loss of deciduous teeth, and teeth extraction may occur with concomitant gingival bleeding and bruising [4]. Various associated symptoms include hemarthrosis, epistaxis, enterohemorrhage, hemothorax, and splenic rupture and thrombosis due to administration of fibrinogen may lead patient mortality. Accompanying gynecological conditions are menorrhagia, menometrorrhagia, spontaneous recurrent abortion, antepartum and postpartum hemorrhage. Interestingly, our patient had no bleeding episode other severe epistaxis in infancy until presenting with hemoperitoneum at the age of 18.
Hemoperitoneum is frequently observed in surgical diseases including blunt abdominal trauma, aneurysmal rupture, malignant tumor in solid organs, pancreatitis, and pancreatic pseudocyst, and complications of inflammatory diseases such as pancreatitis and pancreatic pseudocyst. Occurrence is very rare without a precipitating cause. Significant recent development of diagnostic methods using imaging allows clinicians to identify the causes of hemoperitoneum more easily. However, most commonly the cause remains unknown. Spontaneous (or idiopathic) hemoperitoneum is categorized into hepatic, splenic, vascular, gynecologic, and altered coagulation status based on the cause of the disease [5]. First, the most common causes are hepatic and the majority of these are due to undiagnosed benign and malignant tumors including hepatic adenoma, hemagioma, and hepatoma. Second, splenic cause is very rare and is associated with inflammatory diseases. Third, vascular causes are divided into arterial causes that are mostly due to aneurysm and venous causes that are usually secondary to bleeding from the abdominal vein. Fourth, the gynecologic causes are mainly due to pregnancy, however, nongestational causes including ovarian cystic tumor and hemorrhagic corpus luteal cyst may also present. Finally, coagulative causes are mostly associated with anticoagulation and the cases due to innate absence of coagulation factors though very rare can possibly occur. The present case was a spontaneous hemoperitoneum due to combination of innate defect in coagulation factors and hemorrhagic corpus luteal cyst. Intraperitoneal hemorrhage usually presents as acute abdominal pain and sometimes accompanies symptoms such as abdominal distension, hypotension, syncope, and tachycardia. Exclusion of other causes that may result in acute abdominal pain based on laboratory findings, physical examination and detailed and medical history is essential to treatment. Determining intraperitoneal hemorrhage and its causes by diagnostic imaging including abdominal ultrasonography, abdominal computed tomography, and magnetic resonance imaging are critical to treatment planning. Delay in all blood coagulation tests related to the formation of cellulose including bleeding time (BT), prothrombin time (PT), partial thromboplastin time (aPTT), and reptilase time is observed since the fibrinogen essential in cellulose formation is insufficient in patients with congenital hypofibrinogenemia. Such delay in blood coagulation tests is reversible on administration of normal plasma or concentrated fibrinogen.
The diagnosis of congenital hypofibrinogenemia is confirmed by immunological quantitation of fibrinogen. For treatment of hemoperitoneum in patients with congenital hypofibrinogenemia, conservative treatment may be more effective than prompt laparotomy or diagnostic laparoscopy in which the accurate area of bleeding may not be identified. The fundamentals of conservative treatment are fibrinogen supplementation [67]. Despite the 2-4 day half-life of fibrinogen, blood fibrinogen concentration should be measured on a daily basis due to the inter- as well as occasional intra-patient differences in recovery and maintenance of fibrinogen. FFP is more commonly used since the administration of fibrinogen may cause allergic reaction, hepatitis, thrombosis and rarely formation of antibody against fibrinogen [8]. In the current case, surgical treatment was unavoidable due to persistent bleeding despite conservative treatment.
Prophylactic administration of fibrinogen is not recommended because a patient can have allergic reaction and sometimes hepatitis or antibody formation can be triggered. Furthermore, rare thrombotic events can result from prophylaxis with fibrinogen [27]. In such cases, oral contraceptive pills are helpful to prevent acute bleeding episodes such as hemoperitonium with corpus luteal cystic rupture. Oral pills raise the possibility of thrombotic events, hence gynecologists have to pay attention to such side effects when administering hormonal medication [910].
In conclusion, intra-abdominal hemorrhage due to the rupture of a corpus luteal cyst is a rare but potentially fatal condition. Therefore, gynecologists should recognize the potential of such fatal complication after menarche in all patients with congenital hypofibrinogenemia.
Figures and Tables
References
1. Asselta R, Duga S, Tenchini ML. The molecular basis of quantitative fibrinogen disorders. J Thromb Haemost. 2006; 4:2115–2129.
2. Acharya SS, Coughlin A, Dimichele DM. North American Rare Bleeding Disorder Study Group. Rare Bleeding Disorder Registry: deficiencies of factors II, V, VII, X, XIII, fibrinogen and dysfibrinogenemias. J Thromb Haemost. 2004; 2:248–256.
3. Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood. 2004; 104:1243–1252.
4. Peyvandi F, Haertel S, Knaub S, Mannucci PM. Incidence of bleeding symptoms in 100 patients with inherited afibrinogenemia or hypofibrinogenemia. J Thromb Haemost. 2006; 4:1634–1637.
5. Lucey BC, Varghese JC, Soto JA. Spontaneous hemoperitoneum: causes and significance. Curr Probl Diagn Radiol. 2005; 34:182–195.
6. Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. A United Kingdom Haemophilia Center Doctors' Organisation (UKHCDO) guideline approved by the British Committee for Standards in Haematology. Haemophilia. 2008; 14:671–684.
7. Bolton-Maggs PH, Perry DJ, Chalmers EA, Parapia LA, Wilde JT, Williams MD, et al. The rare coagulation disorders--review with guidelines for management from the United Kingdom Haemophilia Centre Doctors' Organisation. Haemophilia. 2004; 10:593–628.
8. Oruc N, Tokat Y, Killi R, Tombuloglu M, Ilter T. Budd-Chiari syndrome in an afibrinogenemic patient: a paradoxical complication. Dig Dis Sci. 2006; 51:378–380.
9. Bottini E, Pareti FI, Mari D, Mannucci PM, Muggiasca ML, Conti M. Prevention of hemoperitoneum during ovulation by oral contraceptives in women with type III von Willebrand disease and afibrinogenemia. Case reports. Haematologica. 1991; 76:431–433.
10. Castaman G, Ruggeri M, Rodeghiero F. Congenital afibrinogenemia: successful prevention of recurrent hemoperitoneum during ovulation by oral contraceptive. Am J Hematol. 1995; 49:363–364.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download