Abstract
This study assesses the efficacy and safety of a 24-day regimen of drospirenone-containing combined oral contraceptive, and demonstrates that it is an effective and safe option for contraception, releasing symptom of premenstrual dysphoric disorder and acne in Korean women.
Combined oral contraceptive (COC) is a popular and effective method for contraception. Since dose of estrogen or type of progestin component in COCs are associated with side effects and adverse outcomes, especially cardiovascular diseases [12], efforts have been paid to reduce dose of estrogen and change progestin in COCs over the past 50 years. In addition, a new progestin, which is a derivative of progesterone or spironolactone, is now used to minimize androgenic or estrogenic effects [3]. Nevertheless, the rate of COC use is still low in Korea than those in other countries [4], and this may be due to misconceptions and cultural prejudices against COCs. This study was conducted to assess the efficacy and safety of a 24-day COC containing drospirenone (DRSP) 3 mg and ethinyl estradiol 20 µg in Korean women.
This prospective, non-interventional study enrolled patients who were recruited from 68 clinics or hospitals in Korea. Healthy women from 18 to 50 years of age who visited gynecologists for contraception with or without premenstrual dysphoric disorder (PMDD) or acne were invited to join this study. Exclusion criteria were follows: use of DRSP-containing COC before enrollment; presence or history of venous or arterial thrombotic/thromboembolic events (e.g., deep venous thrombosis, pulmonary embolism, myocardial infarction) or cerebrovascular accident; presence or history of prodromi of thrombosis, history of migraine with focal neurological symptoms; diabetes mellitus with vascular involvement; presence of severe or multiple risk factor for venous or arterial thrombosis; pancreatitis or history thereof if associated with severe hypertriglyceridemia; presence or history of severe hepatic disease as long as liver function values have not returned to normal; severe renal insufficiency or acute renal failure; presence or history of liver tumors; known or suspected sex-steroid influenced malignancies; undiagnosed vaginal bleeding; known or suspected pregnancy, hypersensitivity to the active substances or to any of the excipients. This study was approved by the institutional review board for clinical research at each center (NCT00998257).
A 24-day COC containing 3 mg of DRSP and 20 µg of ethinyl estradiol (Yaz, Bayer Healthcare, Seoul, Korea) was used for the study. The study participants were followed-up for six cycle of treatment from July 2009 to November 2011. Several office visits, such as initial visit for enrollment, at least one follow-up visit during the study period, and the last visit after 6 cycles of treatment, were scheduled. The decision on the duration and closure of treatment is up to the discretion of the attending physician.
The primary objective was to assess the efficacy of Yaz in preventing pregnancies. Compliance was evaluated by comparing numbers of taken pills and prescribed pills. Presence of pregnancy was evaluated by pregnancy test (positive/negative). The second objective was to evaluate the effect of Yaz on PMDD, which was diagnosed by the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) [5], and acne. To assess improvement in PMDD and acne, each study participant chose the check box whether their PMDD or acne was much better, better, no change, or worsen. The safety variables were adverse events, which were measured at each follow up visit and final observational point. Symptoms, serious adverse event or not, reason to be considered as severity, time of onset, time of vanishing symptom, outcome, treatment, and relationship with Yaz were recorded.
During the study period, a total of 770 women were enrolled. Among them, 764 completed evaluation for safety, and 762 for efficacy. Six women were excluded due to protocol violation, and two were excluded from analysis due to missing data. Therefore, response rate was 99.2% (764/770).
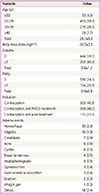
Table 1 presents baseline characteristics of the study participants. Mean age was 28.7±6.6 years old, and the most dominant age group was 20 to 29 years old (59.3%). Mean gravidity and parity were 0.4±0.8 and 0.8±1.1, respectively. In addition, mean amenorrhea period (date of starting combined oral contraceptive date of last menstruation) was 21.1±39.5 days. Indications of COC use were contraception and PMDD treatment (48.2%), only contraception (42.3%), and contraception and acne treatment (15.1%), in order.
During the study period, no pregnancy was reported. Efficacy for PMDD and acne treatment, which were classified into four categories by the study participants, were evaluated (Fig. 1A). For PMDD, proportions of 'much better' (32.5%) and 'better' (59.8%) were higher than those of 'no change' or 'worse.' Similar effects were also shown for acne, showing 'much better' in 26.7% and 'better' in 56.0% (Fig. 1A). In the analysis of factors affecting efficacy and effectiveness rate, duration of use was significantly associated positively with PMDD improvement (P<0.001, data not shown).
In general, compliance for the Yaz was high during the study period, regardless of its indication for use. Proportions of women who had compliance over 90% were 87% for contraception, 74.7% for contraception and PMDD treatment, and 76.5% for contraception and acne treatment (Fig. 1B).
During the study period, 82 adverse events were reported in 62 women through the open question (8.1%, 62/764 subjects). Details of the reported adverse events are shown in Table 1. Most common single adverse event was bleeding in 2.6% (20/764), and followed by vaginitis (1.3%, 10/764), candidiasis (0.9%, 7/764), and acne (0.8%, 6/764) (Table 1). However, no serious adverse event was reported during the study period. In the analysis of factors affecting safety and incidence of adverse events, experience of contraception (P<0.001) and concomitant use of medication (P<0.001) were statistically significant.
Over the past decades, oral contraceptive has been developed with reduction of estrogen dose with advent of new progestins [6]. In particular, Yaz has potential benefits such as better ovarian suppression and cycle control, and more forgiving of forgetfulness, compared to other conventional COCs. Although there is a plenty of studies regarding COC worldwide, Korean women are still reluctant to take COC for contraception or non-contraceptive benefits. This study evaluated efficacy and safety of a 24-day regimen COC containing DRSP, and showed that it was highly effective and safe contraceptive option for Korean women.
No pregnancy was found in the current study during the study period. Although the number of study participants was small and follow-up duration was relatively short, this finding is consistent with a recent paper showing that the Pearl Index was as low as 0.3% with perfect use of COC [7]. A 24-day regimen COC is a very effective contraceptive option in Korean women as in other countries.
In respect of non-contraceptive benefits, our study presented that symptoms related to PMDD or acne were relieved with a 24-day COC use. It has been reported that COCs could improve the symptom of PMDD significantly compared with placebo [8]. Wichianpitaya et al. also reported that the symptom of PMDD was clinically improved after 3 cycles of COC use [9], and moreover, dysmenorrhea has been relieved in over 90% of patients using COC [10]. There are also many studies evaluating a relationship between acne and COC use, and the number or severity of acne lesions was reduced in COC users compared to those in placebo group [11]. In addition, over 90% of both patients and physicians thought Yaz was 'very useful' or 'useful' for all indications in the present study (data not shown).
In the current study, most side effects of Yaz were within predictable range. The most common appealing discomfort was vaginal bleeding, and indeed, it is the most common reason for the discontinuation of oral contraceptive by 6 months [12]. Women who had experienced vaginal spotting during COC use was only 2.6% in the present study, in accordance with a previous study showing that only 0.7% discontinued Yaz because of bleeding over a period of one year [13]. Our findings suggest that side effects during a 24-day regimen COC are not serious, and a 24-day regimen could be an adequate COC for Korean women. In addition, serious adverse events such as venous thromboembolism or arterial thrombotic were not observed during the study period. Although risks of these problems are thought to be minimal in Asian women compared to Western women in general, a larger study population and longer study duration is necessary to draw a concrete conclusion on this issue.
This study has several limitations. First, the duration of study period was short and the number of study participants was small to assess serious adverse outcomes such as cardiovascular events as other previous studies. Second, the method of evaluating efficacy on symptoms of PMDD and acne was subjective. Improvement in symptoms was addressed based on patient's subjective feelings, not on objective measurements. Furthermore, for PMDD, information on baseline physical or psychological symptoms that met DSV-IV criteria was lacking. Third, there was no control (placebo) group in this study. This point can lead to difficulties in exactly evaluating the efficacy and safety of Yaz, and also can result in differences compared to previous clinical trials. Finally, there was no information on whether study participants had ever taken other types of COC before joining this study. In spite of these limitations, this is the first study to address efficacy and safety of a 24-day regimen COC in Korea.
In conclusion, the current study demonstrates that Yaz is an effective and safe contraceptive option which also has non-contraceptive benefits on PMDD and acne in Korean women. A larger and long-term study is warranted to address severe adverse effects related to oral contraceptive use in Korean women in the future.
Figures and Tables
References
1. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009; 339:b2890.
2. Van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009; 339:b2921.
3. Sitruk-Ware R, Nath A. The use of newer progestins for contraception. Contraception. 2010; 82:410–417.
4. Lee DY, Koo YA, Yoon BK, Choi D. Reproductive health characteristics of urban South Korean women. Gynecol Obstet Invest. 2010; 70:154–159.
5. Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology. 2003; 28:Suppl 3. 25–37.
6. Archer DF, Maheux R, DelConte A, O'Brien FB. Efficacy and safety of a low-dose monophasic combination oral contraceptive containing 100 microg levonorgestrel and 20 microg ethinyl estradiol (Alesse). North american Levonorgestrel Study Group (NALSG). Am J Obstet Gynecol. 1999; 181(5 Pt 2):39–44.
7. Trussell J. Contraceptive failure in the United States. Contraception. 2011; 83:397–404.
8. O'Brien PM. The premenstrual syndrome. A review. J Reprod Med. 1985; 30:113–126.
9. Wichianpitaya J, Taneepanichskul S. A comparative efficacy of low-dose combined oral contraceptives containing desogestrel and drospirenone in premenstrual symptoms. Obstet Gynecol Int. 2013; 2013:487143.
10. Robinson JC, Plichta S, Weisman CS, Nathanson CA, Ensminger M. Dysmenorrhea and use of oral contraceptives in adolescent women attending a family planning clinic. Am J Obstet Gynecol. 1992; 166:578–583.
11. Leyden J, Shalita A, Hordinsky M, Swinyer L, Stanczyk FZ, Weber ME. Efficacy of a low-dose oral contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonorgestrel for the treatment of moderate acne: a randomized, placebo-controlled trial. J Am Acad Dermatol. 2002; 47:399–409.
12. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998; 179(3 Pt 1):577–582.
13. Bachmann G, Sulak PJ, Sampson-Landers C, Benda N, Marr J. Efficacy and safety of a low-dose 24-day combined oral contraceptive containing 20 micrograms ethinylestradiol and 3 mg drospirenone. Contraception. 2004; 70:191–198.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


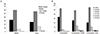
 XML Download
XML Download