Abstract
Objective
To evaluate the learning curve of laparoscopic radical hysterectomy (LRH) for gynecologic oncologists who underwent residency- and fellowship-training on laparoscopic surgery without previous experience in performing abdominal radical hysterectomy (ARH).
Methods
We retrospectively reviewed 84 patients with FIGO (International Federation of Gynecology and Obstetrics) stage IB cervical cancer who underwent LRH (Piver type III) between April 2006 and March 2014. The patients were divided into two groups (surgeon A group, 42 patients; surgeon B group, 42 patients) according to the surgeon with or without ARH experience. Clinico-pathologic data were analyzed between the 2 groups. Operating times were analyzed using the cumulative sum technique.
Results
The operating time in surgeon A started at 5 to 10 standard deviations of mean operating time and afterward steeply decreased with operative experience (Pearson correlation coefficient=-0.508, P=0.001). Surgeon B, however, showed a gentle slope of learning curve within 2 standard deviations of mean operating time (Pearson correlation coefficient=-0.225, P=0.152). Approximately 18 cases for both surgeons were required to achieve surgical proficiency for LRH. Multivariate analysis showed that tumor size (>4 cm) was significantly associated with increased operating time (P=0.027; odds ratio, 4.667; 95% confidence interval, 1.187 to 18.352).
Conclusion
After completing the residency- and fellowship-training course on gynecologic laparoscopy, gynecologic oncologists, even without ARH experience, might reach an acceptable level of surgical proficiency in LRH after approximately 20 cases and showed a gentle slope of learning curve, taking less effort to initially perform LRH.
Laparoscopic techniques have been increasingly performed as a part of the standard surgical armamentarium for early stage cervical cancer patients in many institutions. As a consequence, abdominal radical hysterectomy (ARH) is gradually being replaced by laparoscopic radical hysterectomy (LRH) or robotic radical hysterectomy [12345678]. However, laparoscopic surgery has some limitations such as increased operating time (OT) and longer training period [9]. Two-dimensional views with reduced depth perception, rigid instruments and bad ergonomic circumstances may also result in a surgeon's fatigue and awkward positioning. Thus, LRH is still considered a technically demanding procedure that requires expertise in open surgery and advanced laparoscopic techniques.
Only a few reports analyzing the learning curve for LRH have been published [1011]. However, the participants in these studies were well-trained gynecologic oncologists who were thoroughly familiar with ARH. To date, there has been no study that evaluates the learning curve of LRH performed by surgeons without open counterpart experience. Furthermore, most recent postgraduate gynecologists have had little or no experience with ARH during their training period. Although some urologic surgeons revealed that previous experience with open surgery has little effect on the performance of laparoscopic procedures, there has been no study on the effectiveness of ARH experience on performing LRH [12].
A cumulative sum (CUSUM) chart is widely used for quality control of industrial production lines [13]. A CUSUM chart improves the ability to detect small shifts by charting a statistic that incorporates current and previous data values from the process. Similarly, it can allow researchers to inspect and review the surgical performance and evaluate the learning curve during acquisition of a new practical skill without prior sample size calculation [13]. Using CUSUM analysis, our study aimed to evaluate the learning curve of LRH for gynecologic oncologists who underwent residency- and fellowship-training on laparoscopic surgery without previous experience in performing ARH.
All consecutive patients with 2009 International Federation of Gynecology and Obstetrics (FIGO) stage IB cervical cancer treated at Ajou University Hospital between April 2006 and March 2014 were identified. The patients were divided into two groups (surgeon A group, 42 patients; surgeon B group, 42 patients) according to the surgeon with or without ARH experience. During the residency-training course, both surgeons were trained in laparoscopic surgery and familiar with simple pacemaker operations including benign laparoscopic adnexal surgery and hysterectomy. With respect to the radical hysterectomy, surgeon A (SJ Chang) performed ARH as a surgical assistant and primary surgeon on approximately 100 cases. However, surgeon B (TW Kong) experienced LRH on approximately 50 cases as a surgical assistant during the residency- and fellowship-training course, but no experience on ARH.
All patients were diagnosed with invasive squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma on cervical punch biopsy or conization. The inclusion criteria were as follows: FIGO stage IB cervical cancer without any evidence of parametrial invasion or lymph node (LN) metastasis on preoperative pelvic examination, magnetic resonance imaging and positron emission tomography/computed tomography examinations; and no underlying disease that could influence survival. Patients who were diagnosed with non-squamous cell carcinoma or non-adenocarcinoma, such as clear cell or neuroendocrine tumors, on cervical punch biopsy were excluded. However, two patients, who were diagnosed with squamous cell carcinoma and adenocarcinoma on cervical punch biopsy, had sarcomatoid squamous cell carcinoma and small cell carcinoma in radical hysterectomy specimens. Patients with a history of primary radiotherapy or neoadjuvant chemotherapy were also excluded. Prior surgery and patients' body weight were not included in the considerations of the contraindications for the laparoscopic approach. Approval was given by the Ajou University Hospital Institutional Review Board. All eligible patients underwent Piver type III RH with pelvic and/or para-aortic lymphadenectomy. Pelvic lymphadenectomy alone was performed in patients with FIGO stage IB1 ≤2 cm in size without pelvic LN metastasis [14]. All participants signed the written informed consent. All surgeries were performed with a resident as the first assistant. LRH was performed based on the technique described by Nam et al. [3].
We recorded parameters including clinical characteristics (age, parity, body mass index [BMI], tumor markers, mean OT, estimated blood loss [EBL], hospital stay, return of bowel motility, duration of follow up, adjuvant therapy, recurrence), histopathologic characteristics (histology, FIGO stage, tumor size, pathologic risk factors, number and status of LNs removed), and perioperative morbidities. OT was recorded from the beginning of the skin incision to the completion of operative wound closure. Blood loss was estimated from the amount collected in the suction device. Complications were defined as any event during and after surgery that required further surgical procedure, interventional radiology or rehabilitation therapy.
We used a CUSUM analytical method similar to that described by Bokhari et al. [15]. The CUSUM is the running CUSUM of differences between measured individual values and the target. The mean OT for each surgeon was used as the target. We tried to detect "out-of-control" signals in the statistical process control. In our analysis, the decision interval and reference value had to be decided to draw a CUSUM chart. We used the decision interval of 1 standard deviation and the reference value of 0.5. In the CUSUM charts, the one-sided upper and lower CUSUMs accumulate deviations from the target that are greater than the reference value and reset to zero upon becoming negative. If either of the one-sided upper CUSUM or the lower CUSUM exceeds the decision interval (denoted as upper decision bound or lower decision bound in our CUSUM charts), the process is considered "out of control." We used the CUSUM technique to analyze the total OT after placing our cases in chronological order based on operation dates. Analyses were done using R 2.14.1 software with package 'qcc.'
We performed Mann-Whitney U-tests, linear by linear association, Fisher's exact test, Spearman's rho, and binary logistic regression as appropriate. Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). A threshold of P<0.05 was considered statistically significant.
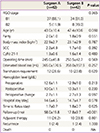
Table 1 compares the clinical characteristics for surgeons A and B. There were no significant differences between the two groups with regard to age, BMI, tumor markers, FIGO stage, OT, perioperative hemoglobin change, transfusion requirements, hospital stay, return of bowel motility, adjuvant therapy and recurrence, but the mean follow-up period in the surgeon B group was shorter than in the surgeon A group (58.0 versus 17.8 months, P<0.001). The mean OT was similar in both groups (245.0 minutes in the surgeon A group versus 250.5 minutes in the surgeon B group, P=0.569).
There were no significant differences in histopathologic type, grade, pathologic tumor size, length of parametrium and vaginal cuff, and pathologic risk features between the two groups (Table 2). The mean numbers of pelvic and para-aortic LNs retrieved were 22.0 and 8.9 in the surgeon A group, and 23.0 and 10.0 in the surgeon B group, respectively (pelvic LNs, P=0.189; para-aortic LNs, P=0.403). The mean pathologic tumor size was 27.0 mm (range, 10 to 60 mm) in the surgeon A group and 29.0 mm (range, 10 to 70 mm) in the surgeon B group. Adequate margins of paracervical and vaginal cuff resection were achieved through LRH (Fig. 1).
There were no significant differences in perioperative morbidities between the two groups (Table 3). In the surgeon A group, 3 cases of ureteral injury, 1 case of bladder injury, 3 cases of lymphocyst and 1 case of vesicovaginal fistula were noted. There were 1 case of ureteral injury and 2 cases of lymphocyst in the surgeon B group. Neither incisional hernia nor port-site metastasis was detected. There was no case that required conversion to open surgery.
In the regression analysis (Fig. 2), OT steeply decreased with operative experience in surgeon A (Pearson correlation coefficient=-0.508, P=0.001). Surgeon B, however, showed a gentle slope of learning curve (Pearson correlation coefficient=-0.225, P=0.152). Although some variations in the learning process were shown, approximately 18 cases for both surgeons were required to achieve surgical proficiency in LRH (Fig. 3). Forty-two patients in the surgeon A and B group were divided into the first 18 cases (phase I) and the second 24 cases (phase II).
There were no significant differences in clinico-pathologic outcomes and perioperative morbidities between phase I and phase II patients in both groups (Table 4). However, OT decreased significantly in the second phase of the series (265.6 vs. 229.5 minutes in the surgeon A group, P=0.013; 269.3 vs. 236.4 in the surgeon B group, P=0.012).
Table 5 shows some clinico-pathologic variables in relation to increased OT of LRH. Increased OT means that the OT is longer than the mean OT of both groups (247.7 minutes). Forty-eight patients in phase II of both groups were analyzed. Univariate analysis showed significantly longer OT in patients whose tumor size was >4 cm (P=0.022). The performance of para-aortic lymphadenectomy, BMI, and risk factors for scarring, such as previous conization and cesarean section status, were not significant. According to the multivariate analysis, tumor size (>4 cm) was significantly related to the increased OT (P=0.027; OR, 4.667; 95% CI, 1.187 to 18.352).
In the era of laparoscopy, trainees first act as the camera holder, and then selectively perform simple pacemaker operations including laparoscopic hysterectomy or adnexectomy, and then move onto more advanced laparoscopic surgery. Recently, postgraduate gynecologic oncologists in many institutions have little or no experience with ARH during their training period. However, an early integration of surgical trainees into performing laparoscopic surgery is an important factor in acquiring the specific psychomotor skills and eye-hand coordination. Furthermore, LRH is a step-by-step procedure which includes retroperitoneal lymphadenectomy, development of the pelvic sidewall including the paravesical and pararectal spaces, bladder takedown, ligation of the uterine artery, dissection of the ureter, posterior and lateral dissection, and vaginal resection. In addition, the ability to view the magnified anatomy during laparoscopy may be beneficial for trainees learning to perform LRH. Therefore, it is worthwhile to evaluate the learning curve analysis of LRH for gynecologic oncologists without open counterpart experience who underwent residency- and fellowship-training on laparoscopic surgery.
In total LRH, Pomel et al. [1] reported that the mean OT for the first 25 cases was 290 minutes, and 226 minutes for the last 25 cases, while in laparoscopic assisted radical vaginal hysterectomy, Steed et al. [16] found that the OT of the last 30 patients was 20 to 30 minutes shorter than that of the first 30 patients. We also found that the mean OT in phase II patients was approximately 30 to 40 minutes shorter than that of phase I patients. It is certainly true that speed does not equate to surgical proficiency. Surgical learning curve assessment generally measures the complication rate as well as the OT. However, we did not observe a trend in either increase or decrease of perioperative morbidities and EBL. Also, the number of retrieved LNs did not change with experience in this study. Moreover, visual assessment of EBL was not only subjective data, but also tendentious estimates. Therefore, providing that a surgeon performing a new procedure shows acceptable histopathologic outcomes and perioperative morbidities, the OT is a fair reflection of the entire learning process. Thus, we assessed the OT for the learning curve analysis.
To date, there have been two learning curve studies on LRH [1011]. The surgeon in the first study had completed a fellowship in gynecologic oncology [10]. Initially one surgeon performed the entire operation, and later in the series, the second surgeon began performing more of the operation under supervision. Thus, this study did not evaluate the learning curve of LRH performed by a single surgeon. The participant in another learning curve analysis was familiar to ARH, but had no experience with benign laparoscopic surgery [11]. Our results demonstrated that the learning curve in this study was much shorter than that in the published data. The magnified view of the retroperitoneal anatomy might enable laparoscopic trainees to learn LRH over a relatively shorter training period, particularly making it easier to dissect the ureter and coagulate small vessels. In addition to the early laparoscopic training and magnified view, the vaginal cuff was resected and closed by the transvaginal route, which might allow shorter OT.
To evaluate the surgical proficiency in LRH, we used CUSUM methods, which provide an objective way to statistically define the process of acquiring a new skill, instead of using the common practice of dividing the study population chronologically into arbitrary fractions for analysis. The CUSUM method was used to analyze the learning curve for LRH in a previous study [11]. Failure was defined as surgery that took longer than the average OT of LRH (287 minutes), which was longer than that of our study (245.0 minutes in the surgeon A group vs. 250.5 minutes in the surgeon B group) even though para-aortic lymphadenectomy was performed in our study. Furthermore, in that study, a failure rate below 40% was considered as acceptable, and a failure rate above 60% as unacceptable. Researchers in that study recognized that the lack of tutors and limited experience with laparoscopic surgery in benign gynecology might have contributed to the longer learning time. Our study evaluated the surgical proficiency in LRH, using the mean OT in each surgeon as the target (Fig. 3). To make the criteria of the acceptable range narrow, we used the decision interval of 1 standard deviation (Fig. 3). Our studies demonstrated that the OT of LRH in phase I of both groups (265.6 minutes in the surgeon A and 269.3 minutes in the surgeon B group) at the beginning of both surgeons' learning curve was longer than the mean OT (245.0 minutes in the surgeon A and 250.5 minutes in the surgeon B group). Surgeon A was able to achieve a mean OT of less than 265.6 minutes after about 18 cases and his mean OT continued to decrease to the mean OT in phase II of surgeon A (229.5 minutes). Surgeon B was also surgically proficient after about 18 cases and his mean OT tended to decrease to the mean OT of phase II (236.4 minutes). The learning curve of surgeon A started at 5 to 10 standard deviations of mean OT and afterward OT of surgeon A continued to decrease to the mean OT with a steep slope. This slower starting time and steep slope collectively indicate that performance of LRH for surgeon A was initially difficult and it took some effort to be accustomed to the laparoscopic environment. On the contrary, the learning curve of surgeon B started at 2 standard deviations of mean OT and afterward OT of surgeon B continued to decrease to the mean OT with a gentle slope, which suggests that experience in LRH as a surgical assistant plays significant effect to allow the surgeon to become accustomed to performing LRH. Therefore, it might be possible to generalize the learning curve obtained in this study to a surgeon who has had considerable experience with gynecologic laparoscopic surgery, particularly LRH, during the residency- and fellowship-training period.
In the current study, the learning period for LRH showed that it took less than 20 cases to achieve a turning point in OT, reflecting the improvements in visualization of the anatomy and the early laparoscopic training. In addition, the mean OT in our study was shorter than that of the previous learning curve study because of the time saved during the vaginal cuff resection and closure. However, several risk factors can affect the OT. We postulated that BMI, bulky tumor size, the performance of para-aortic lymphadenectomy, and other risk factors that result in difficult tissue dissection including previous conization or cesarean section status might be associated with longer OT. Even after achieving surgical proficiency, a longer OT for LRH was observed in 2 out of 8 patients with a tumor size >4 cm in the surgeon A group. In the surgeon B group, there were 5 patients with a bulky tumor who showed a longer OT. Also, the multivariate analysis showed that the bulky cervical mass (>4 cm) served as a significant independent variable for longer OT. With respect to the learning curve of LRH, previous experience with ARH might have little effect on the performance of LRH. However, the risk of open conversion still exists. Therefore, it could be necessary to manage such inevitable complications of laparoscopic surgery via the open approach.
There were some limitations in our study. To adequately analyze the learning curve for LRH in early stage cervical cancer, it might be necessary to evaluate a higher number of cases than in our study. Also, the follow-up period in surgeon B group was too short to make any conclusive remarks on recurrence and survival. In addition, there are variations in surgeons' skill and training course, which may result in different outcomes from our study. Despite the known limitations, we expect that a laparoscopic trainee through residency- and fellowship-training course can achieve surgical proficiency in LRH after a relatively small number of cases, compared to the previous study on the learning curve of LRH, even without ARH experience.
In conclusion, previous experience with ARH might have little effect on the performance of LRH. We demonstrate that gynecologic oncologists after completing a residency- and fellowship-training course including laparoscopy, even without open counterpart experience, can achieve an acceptable level of surgical proficiency in LRH after approximately 20 cases. Furthermore, early experience and familiarity with the retroperitoneal anatomy under laparoscopic vision might allow gynecologic oncologists to become more accustomed to performing LRH, showing a gentle slope of learning curve.
Figures and Tables
 | Fig. 2Regression analysis of operating time in surgeon A (Pearson correlation coefficient=-0.508, P=0.001) and B (Pearson correlation coefficient=-0.225, P=0.152). |
 | Fig. 3Learning curve for laparoscopic radical hysterectomy using cumulative sum charts. (A) Surgeon A and (B) surgeon B. |
Table 2
Histopathologic characteristics between the two surgeons
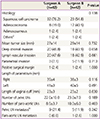
Data are presented as number (%) or mean±standard deviation.
LN, lymph node.
a)Two patients who were diagnosed with squamous cell carcinoma and adenocarcinoma on cervical punch biopsy had sarcomatoid squamous cell and small cell carcinoma in radical hysterectomy specimens; b)The number of positive LNs (range, 1 to 6).
References
1. Pomel C, Atallah D, Le Bouedec G, Rouzier R, Morice P, Castaigne D, et al. Laparoscopic radical hysterectomy for invasive cervical cancer: 8-year experience of a pilot study. Gynecol Oncol. 2003; 91:534–539.
2. Puntambekar SP, Palep RJ, Puntambekar SS, Wagh GN, Patil AM, Rayate NV, et al. Laparoscopic total radical hysterectomy by the Pune technique: our experience of 248 cases. J Minim Invasive Gynecol. 2007; 14:682–689.
3. Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012; 23:903–911.
4. Choi CH, Lee JW, Lee YY, Kim HJ, Song T, Kim MK, et al. Comparison of laparoscopic-assisted radical vaginal hysterectomy and laparoscopic radical hysterectomy in the treatment of cervical cancer. Ann Surg Oncol. 2012; 19:3839–3848.
5. Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008; 109:86–91.
6. Cantrell LA, Mendivil A, Gehrig PA, Boggess JF. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a 3-year experience. Gynecol Oncol. 2010; 117:260–265.
7. Sert MB, Abeler V. Robot-assisted laparoscopic radical hysterectomy: comparison with total laparoscopic hysterectomy and abdominal radical hysterectomy; one surgeon's experience at the Norwegian Radium Hospital. Gynecol Oncol. 2011; 121:600–604.
8. Tinelli R, Malzoni M, Cosentino F, Perone C, Fusco A, Cicinelli E, et al. Robotics versus laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: a multicenter study. Ann Surg Oncol. 2011; 18:2622–2628.
9. Vickers AJ, Savage CJ, Hruza M, Tuerk I, Koenig P, Martinez-Pineiro L, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol. 2009; 10:475–480.
10. Reade C, Hauspy J, Schmuck ML, Moens F. Characterizing the learning curve for laparoscopic radical hysterectomy: buddy operating as a technique for accelerating skill acquisition. Int J Gynecol Cancer. 2011; 21:930–935.
11. Hwang JH, Yoo HJ, Joo J, Kim S, Lim MC, Song YJ, et al. Learning curve analysis of laparoscopic radical hysterectomy and lymph node dissection in early cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2012; 163:219–223.
12. Stolzenburg JU, Rabenalt R, Do M, Horn LC, Liatsikos EN. Modular training for residents with no prior experience with open pelvic surgery in endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2006; 49:491–498.
13. Williams SM, Parry BR, Schlup MM. Quality control: an application of the cusum. BMJ. 1992; 304:1359–1361.
14. Bae JW, Lee JH, Choi JS, Son CE, Jeon SW, Hong JH, et al. Laparoscopic lymphadenectomy for gynecologic malignancies: evaluation of the surgical approach and outcomes over a seven-year experience. Arch Gynecol Obstet. 2012; 285:823–829.
15. Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011; 25:855–860.
16. Steed H, Rosen B, Murphy J, Laframboise S, De Petrillo D, Covens A. A comparison of laparascopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol Oncol. 2004; 93:588–593.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download