Abstract
Objective
The purpose of this study was to investigate the expression of the D6 decoy receptor that can bind chemokines and target them for degradation, resulting in inhibition of inflammation in placentas from preeclamptic and normal pregnancies.
Methods
The current study was carried out in 35 pregnant women (23 patients with preeclampsia and 12 healthy, normotensive pregnant women) during the third trimester of pregnancy. The expressions of D6 decoy receptor in the placenta were determined with real time reverse transcriptase polymerase chain reaction and western blotting.
Results
The mRNA and protein of D6 decoy receptor were detected in all of placentas from preeclamptic and normal pregnancies. Placental D6 decoy receptor mRNA expression was significantly lower in patients with preeclampsia than in patients with normal pregnancies. Western blot analyses revealed decreased protein expression in cases of preeclampsia.
Although the pathophysiology of preeclampsia remains unknown, it is generally accepted that increased maternal systemic inflammatory responses play a major role in its clinical features [1]. Advancing gestation is characterized by a progressive increase in inflammatory response in both normal and preeclamptic pregnancy; however, preeclampsia is associated with more vigorous systemic inflammatory responses than normal pregnancy [23]. It has been hypothesized that the exaggerated systemic inflammation seen in preeclampsia may result from decompensation of one or more maternal protective systems [4].
Chemokines control leukocyte recruitment by activating intracellular signaling pathways through G-protein coupled chemokine receptors [5]. Chemokines also bind to decoy receptors, which are structurally related to signaling receptors but are unable to activate transduction events [6]. The D6 decoy receptor, which shares 30% to 35% sequence identity with signaling chemokine receptors but does not support known signaling functions, recognizes and scavenges a wide spectrum of CC inflammatory chemokines [7]. In skin, gut and lungs, the D6 decoy receptor is expressed on endothelial cells of lymphatic afferent vessels while in placenta, it is expressed by the invading extravillous trophoblast and on the apical side of syncytiotrophoblast cells, at the interface between maternal and fetal circulation [89]. D6 decoy receptor -/- mice used in a model of skin inflammation showed increased inflammatory chemokine levels and an exaggerated inflammatory response, suggesting that the D6 decoy receptor exercises some control over inflammatory response [10]. Moreover, exposure of D6 decoy receptor -/- pregnant mice to lipopolysaccharide (LPS) or antiphospholipid autoantibodies results in higher levels of inflammatory CC chemokines and increased leukocyte infiltrate in placental tissue and increased rates of fetal loss [9]. Therefore, it is implied that the placental D6 decoy receptor plays a role in dampening inflammatory response [9]. However, to date there are no studies evaluating the role of placental D6 decoy receptor in preeclampsia.
The purpose of this study was to investigate the expression of the D6 decoy receptor in placentas from normal and preeclamptic pregnancy.
Placental tissue was obtained from 35 women who gave a birth at Korea University Medical Center. Twenty-three placentas from preeclamptic women, and 12 normal placentas were included in the analysis. A central area (1cm3) of placental tissue on the maternal side was dissected from each sample, and the maternal decidua was removed. Vigorous washing of the maternal blood from the samples was performed with saline and tissues were immediately frozen in liquid nitrogen and stored until use.
Preeclampsia was defined as maternal blood pressure of ≥140/90 mmHg recorded on two occasions separated by six hours after 20 weeks of gestation without a previous history of hypertension, and proteinuria (≥300 mg/24 hours urine or ≥1+ in dipstick test).
This study was approved by the institutional review board of Korea University Medical Center and informed consent was obtained from all patients.
RNA extraction and purification were done using an RNeasy mini kit (Qiagen, Valencia, CA, USA) as described in the manufacturer's protocol. The concentration of RNA was measured using a spectrophotometer (DU530, Beckman, Fullerton, CA, USA). The total RNA sample (2 µg/sample) using the SuperScript III First-Strand Synthesis System for reverse transcriptase (RT) polymerase chain reaction (PCR) kit (Invitrogen, Milan, Italy) were used in a 20 µL scale to generate cDNA. RNA was reverse-transcribed at the following conditions: 25 mM MgCl2, 10 mM dNTP mix, 10 × RT buffer, 0.1 M DTT, 200 U of SuperScript III (Invitrogen), 40U of RNaseOut, 50 µM oligo d(T) primers in a final volume of 20 µL. The reaction was run at 65℃ for 5 minutes and 50℃ for 50 minutes, and then the enzyme was heat inactivated at 85℃ for 5 minutes. Four microliter of reaction products were used for real-time PCR reaction.
Real-time PCR was used to quantify D6 decoy receptor. This expression was normalized using the GAPDH housekeeping gene product as an endogenous reference. The primers and probes were designed for human D6 decoy receptor using Primer Express 2.0 (Applied Biosystems, Foster City, CA, USA). D6 decoy receptor mRNA levels were quantified using TaqMan RT-PCR with an ABI 7700 system (Applied Biosystems). Gene-specific probes and primer pairs for D6 decoy receptor (Assays-on-Demand, Hs00174299_m1; Applied Biosystems) was used. For each probe/primer set, a standard curve was generated, which was confirmed to increase linearly with increasing amounts of cDNA. The amplification conditions were 2 minutes at 50℃, 10 minutes at 95℃, and a two-step cycle of 95℃ for 15 seconds and 60℃ for 60 seconds for a total of 45 cycles.
Total tissues lysates were prepared by homogenization. The tissues was obtained in buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% glycerol, 1% Triton X-100, a mixture of protease inhibitors (aprotinin, phenylmethylsulfonyl fluoride, and sodium orthovanadate). The extracted protein concentration was measured according to the method of Bradford. Equal amounts of total protein were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane (Hybond-P, Amersham Biosciences, Piscataway, NJ, USA). After blocking (tris-buffered saline, 0.1% Tween 20) at 4℃ overnight, the membranes were incubated with primary Ab's for anti human D6 decoy receptor (dilution 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation, the blots were washed (tris-buffered saline 0.1% Tween 20) and incubated with anti goat secondary Ab's linked to horseradish peroxidase (dilution 1:2000; Bio-Rad Laboratories, Hercules, CA, USA). Immunoreactive proteins were visualized by chemiluminescence using SuperSignal West Dura Extended Duration Substrate (Pierce Chemical, Rockford, IL, USA) and signals were detected on X-ray film. Signals in the linear range of the film were digitized, and densitometry was performed using IMT-i-Delta (Image and Microscope Technology, Daejeon, Korea).
Tissue sections (2 µm) were fixed with 4% paraformaldehyde for 10 minutes, blocking solution (phosphate-buffered saline containing 3% bovine serum albumin and 0.02% Triton X-100) was applied for 30 minutes, and anti human D6 decoy receptor (dilution 1:200, Santa Cruz Biotechnology) was added for 1 hour at room temperature. After washing, the cells were exposed to Alexa-488-conjugated anti-mouse IgG (dilution 1:500, Invitrogen, CA, USA) for 30 minutes at room temperature, and their nuclei counterstained with Hoechst 33342 (dilution 1:5000, Invitrogen) for immunofluorescence. Confocal microscopy (LSM 700, Zeiss, Wetzlar, Germany) was used for the distribution of D6 decoy receptor.
Clinical results were reported as mean±SD for continuous variables and as a percentage for categorical variables. Clinical characteristics among groups were compared using the Mann-Whitney U-test and Fisher' exact test, when the variables were continuous and categorical, respectively.
Analysis of variance with a covariant (ANCOVA) using gestational age as covariant was used to test the differences in placental expression of D6 decoy receptor between normal pregnancy and preeclampsia. They were analyzed using the Mann-Whitney U-test. P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
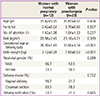
The characteristics of the study subjects, according to presence or absence of preeclampsia, are presented in Table 1. No significant differences in age, parity, number of abortion, and body mass index were observed between women with normal pregnancy and women with preeclampsia. Otherwise, gestational age at delivery and birth weight were different between the two groups. Distributions of neonatal gender, delivery mode, and delivery with labor did not substantially vary between the two groups.
Immunofluorescence stain was carried out to evaluate localize D6 decoy receptor in placentas from normal and preeclamptic pregnancy. Representative micrographs are shown in Fig. 1. The D6 decoy receptor expression was lower in preeclamptic placenta (B) compared to normal placenta (A).
Fig. 2 demonstrates mRNA expression of the placental D6 decoy receptor in preeclamptic and normal placentas. The mRNA D6 decoy receptor was detected in all of placentas from preeclamptic and normal pregnancies. mRNA expression of placental D6 decoy receptor was significantly lower in preeclampsia than in normal pregnancy.
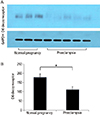
The results of western blot analyses are presented in Fig. 3. Protein expression of the D6 decoy receptor was detected in placentas from both preeclamptic and normal pregnancies with a band detected at approximately 90 kD. Protein expression of the D6 decoy receptor was lower in placentas from preeclamptic pregnancies when compared to that in normal placentas (Fig. 3A). The data from all placentas are shown in Fig. 3B. Densitometric studies have also shown that protein expression of the D6 decoy receptor was significantly lower in placentas from preeclamptic pregnancies than those in normal placentas (P<0.05) (Fig. 3B).
To our knowledge, this is the first study to explore the expression of the D6 decoy receptor in placentas from preeclamptic pregnancies. The results of this study suggest that the expression of the D6 decoy receptor in preeclamptic placentas is significantly lower than that in normal placentas. However, it remains unclear whether the decrease of placental D6 decoy receptor results in the development of preeclampsia or enhances the already heightened inflammatory response observed in women with preeclampsia.
Macrophages near the fetal-maternal interface are associated with placental development, angiogenesis, and decidual remodeling. However, their excessive recruitment is reported to inhibit trophoblast invasion [1112], which may ultimately result in the development of preeclampsia. There is an increased presence of aberrantly-activated macrophages in placentas from preeclamptic pregnancies [11]. Therefore, the mechanism of recruitment of activated macrophages to the maternalfetal interface may be central to the etiology of preeclampsia [13]. However, the trigger for the activation of monocytes/macrophages in preeclampsia remains unknown. Chemokines including eotaxin, macrophage-derived chemokine, monocyte chemoattractant protein (MCP)-3, and macrophage inflammatory protein (MIP)-1β, all of which bind to the D6 decoy receptor, are known to attract monocytes/macrophages and their expressions in endometrium are maintained during pregnancy [14]. The inappropriate control of chemokines in decreased expression of the D6 decoy receptor may result in a significant increase in infiltrating macrophages in placental tissue [9]. Therefore, it is possible that a decrease in the placental D6 decoy receptor may contribute to preeclampsia via the excessive recruitment of macrophages to the fetal-maternal interface. However, D6 decoy receptor -/- mice showed normal placental development and fertility indices, suggesting that its function lies not in the maintenance of homeostatic conditions but rather in the control of placental inflammation of a different origin [9].
Syncytiotrophoblast microparticles (STBMs), the results of turnover and renewal of the syncytial surface of the chorionic villi [3], circulate in normal third trimester pregnancies but are present at significantly higher levels in cases of preeclampsia [1516]. STBMs not only directly damage endothelial cells but also stimulate systemic inflammatory responses in normal and preeclamptic pregnancies [17]. STBMs bind to monocytes and can stimulate the production of TNF-α [18] which in turn can induce the secretion of monocyte/macrophage-attracting chemokines including regulated on activation normal T cell expressed and secreted (RANTES) in invasive trophoblast cells [14] and MCP-1 in invasive trophoblast cells and deciduas [1319]. Therefore, it is possible that a decrease in placental D6 decoy receptors results in an inappropriate response to systemic inflammation caused by STBMs and contributes to an up-regulation of the systemic inflammatory response in preeclampsia.
Maternal infections are also thought to be associated with preeclampsia. For example, active maternal periodontal disease during pregnancy has been found to be associated with an increased risk for the development of preeclampsia [20]. In addition, women with preeclampsia have been found to have increased levels of pathogenic placental microbes compared to controls [21]. Recently, a systematic review of epidemiologic studies revealed that the presence of any infection was associated with a two-fold higher risk of preeclampsia [22]. In particular, exposure to LPS in D6 decoy receptor-/- pregnant mice was shown to result in higher levels of chemokines and increased leukocyte infiltrate in the placenta, causing an increased rate of fetal loss [9]. LPS has been also used as an in vitro stimulus for investigating the inflammatory response in preeclampsia [2324]. Therefore, a decrease in D6 decoy receptors, representing the inappropriate function of dampening placental inflammation, may make an important contribution to the exaggerated systemic inflammation seen in preeclampsia caused by physiological (STBMs) or pathological (infections) conditions.
Labor itself can influence the expression of placental D6 decoy receptor through alteration of chemokines. However, in this study, the expression of D6 decoy receptor was not different between placentas from non-laboring women and placentas from laboring women (data not shown). Moreover, there was no difference in placental expression of D6 decoy receptor between non-severe and severe preeclampsia (data not shown).
Limitations of the current study include differences in gestational age between normal and preeclamptic pregnancies (normal pregnancy vs. preeclampsia, 38.30±1.49 vs. 33.66±3.34 weeks, P<0.001). It is possible that the difference in D6 expression between placentas from preeclamptic and normal placentas was caused by this disparity, but there are currently no published studies evaluating the expression of placental D6 according to gestational age. This may be due to the difficulty associated with sampling placental tissues of less than 30 weeks gestational age from healthy placentas without obstetric compromise. Although ANCOVA revealed that placental D6 expression was significantly lower in preeclampsia than in normal pregnancy after controlling for gestational age (P<0.05), further study is required to investigate the expression of placental D6 considering gestational age.
The present study also did not investigate chemokines that interact with D6. There is currently conflicting evidence whether chemokines are increased in preeclampsia. Jonsson et al. [25] reported that there were no differences in serum levels of MIP-1α, MIP-1β, MCP-1, eotaxin, and RANTES between cases of preeclampsia and normal pregnancies. However, other authors have reported that plasma levels of MCP-1 were increased in preeclampsia [26] and that the RANTES gene was up-regulated in preeclamptic placentas by DNA array [27]. Taken together, the fact that monocyte/macrophage recruitment at the maternal-fetal interface, which is primarily controlled by chemokines, plays a role in the etiology of preeclampsia, further studies are necessary to evaluate the roles of chemokines in the pathophysiology of preeclampsia.
In conclusion, the expression of the placental D6 decoy receptor was significantly lower in preeclamptic pregnancies than in normal pregnancies. Further studies are needed to clarify the underlying mechanisms that link decreased expression of placental D6 decoy receptor and preeclampsia.
Figures and Tables
Fig. 1
Placental expression of D6 decoy receptor (white arrows) by immunofluorescence in normal (A) and preeclamptic (B) placenta (×200).

Fig. 2
mRNA expression of the placental D6 decoy receptor in preeclamptic and normal placentas. Expression was normalized using the GAPDH housekeeping gene product as an endogenous reference. Data were analyzed using ANCOVA (analysis of variance with a covariant) with gestational age as covariant. *P<0.05.
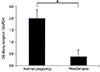
Fig. 3
Western blot analysis for protein expression of placental D6 decoy receptor from preeclamptic and normal placentas. (A) Results from a representative experiment. Bands of almost identical intensity for GAPDH are also shown for each experiment. (B) Mean±standard error of the mean of densitometric numbers was calculated by densitometry. Data were analyzed using ANCOVA (analysis of variance with a covariant) with gestational age as covariant. *P<0.05.
References
1. Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response: a review. Placenta. 2003; 24:Suppl A. S21–S27.
2. Brewster JA, Orsi NM, Gopichandran N, McShane P, Ekbote UV, Walker JJ. Gestational effects on host inflammatory response in normal and pre-eclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2008; 140:21–26.
3. Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006; 11:309–316.
4. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999; 180(2 Pt 1):499–506.
5. Nibbs RJ, McLean P, McCulloch C, Riboldi-Tunnicliffe A, Blair E, Zhu Y, et al. Structure-function dissection of D6, an atypical scavenger receptor. Methods Enzymol. 2009; 460:245–261.
6. Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006; 6:907–918.
7. Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, et al. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol. 2004; 172:4972–4976.
8. Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001; 158:867–877.
9. Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, et al. Protection against inflammation-and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A. 2007; 104:2319–2324.
10. Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005; 35:1342–1346.
11. Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, et al. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999; 20:229–233.
12. Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod. 2005; 73:237–243.
13. Renaud SJ, Sullivan R, Graham CH. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta. 2009; 30:313–319.
14. Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004; 89:6155–6167.
15. Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998; 105:632–640.
16. Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002; 186:158–166.
17. Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007; 76:61–67.
18. Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007; 178:5949–5956.
19. Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006; 168:445–452.
20. Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003; 101:227–231.
21. Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007; 78:670–676.
22. Rustveld LO, Kelsey SF, Sharma R. Association between maternal infections and preeclampsia: a systematic review of epidemiologic studies. Matern Child Health J. 2008; 12:223–242.
23. Luppi P, Deloia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006; 118:268–275.
24. Beckmann I, Efraim SB, Vervoort M, Visser W, Wallenburg HC. Tumor necrosis factor-alpha in whole blood cultures of preeclamptic patients and healthy pregnant and nonpregnant women. Hypertens Pregnancy. 2004; 23:319–329.
25. Jonsson Y, Ruber M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006; 70:83–91.
26. Yarim GF, Karahan S, Nisbet C. Elevated plasma levels of interleukin 1 beta, tumour necrosis factor alpha and monocyte chemotactic protein 1 are associated with pregnancy toxaemia in ewes. Vet Res Commun. 2007; 31:565–573.
27. Heikkila A, Tuomisto T, Hakkinen SK, Keski-Nisula L, Heinonen S, Yla-Herttuala S. Tumor suppressor and growth regulatory genes are overexpressed in severe early-onset preeclampsia: an array study on case-specific human preeclamptic placental tissue. Acta Obstet Gynecol Scand. 2005; 84:679–689.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download