Abstract
Objective
To evaluate whether smoking is a risk factor for female sexual dysfunction (FSD) and to determine the relationship between the cumulative smoking dose and FSD in premenopausal women.
Methods
The study population consisted of sexually active premenopausal women. The frequency of FSD and female sexual function index (FSFI) total score were evaluated according to the smoking status (never/former and current smokers). Evaluation of sexual function was done using FSFI questionnaire, and women with FSFI score of ≤26.55 were considered to have FSD. In current smokers, sexual function was also evaluated according to the cumulative smoking dose and nicotine dependency.
Results
A total of 900 women were included, and the frequency of current smokers and the frequency of FSD were 62 (6.9%) and 496 (55.1%), respectively. In current smokers, the frequency of FSD was significantly higher and the median total FSFI score was significantly lower than in never/former smokers, and this difference of FSD remained significant after adjustment for confounding variables. Among current smokers, the cumulative smoking dose (pack-years) and the total FSFI score showed negative correlation, in which increased cumulative smoking dose was associated with lower total FSFI score (r=-0.278, P<0.05). In terms of nicotine dependency, the total FSFI score of moderately to heavily nicotine dependent smokers was significantly lower than that of lightly dependent smokers.
Female sexual dysfunction (FSD) is defined as recurrent or persistent deficiency in sexual desire and arousal, difficulty or absence of reaching orgasm and genital pain [1]. FSD is affected by multiple factors such as anatomical, physiological, psychological and social factor, and in consequence, it can lead to decreased quality of life [2]. In United States, the prevalence of FSD is 43% in 18- to 59-year-old women, based on a population study [3]. The prevalence of FSD in 18- to 52-year-old Korean women is reported as 43.5% [4], and in a study, 37% of 40- to 80-year-old women had decreased sexual pleasure and 31% had difficulty in achieving an orgasm [56].
Cigarette smoking is a worldwide public health problem, which is related to cardiovascular disease (e.g., peripheral vascular disease, stroke, coronary heart disease, hypertension), respiratory disease, and cancer [7]. In terms of sexual dysfunction, smoking is well known as one of the risk factors of male sexual dysfunction [689]. In men, the association between smoking and impairment of sexual function, especially erectile dysfunction, has been extensively studied, and the positive dose-dependent relationship between smoking and erectile dysfunction has been consistently reported [1011]. However, relatively few studies have addressed the relationship between smoking and sexual dysfunction in women, and the results were inconsistent [121314]. In addition, there is a paucity of information on dose-dependent relationship between cigarette smoking and FSD.
The objective of this study was to evaluate whether smoking is a risk factor for FSD and to determine the relationship between the cumulative smoking dose and sexual dysfunction in Korean premenopausal women.
The study population consisted of consecutive women who visited Seoul National University Hospital Center for Health Promotion and Optimal Aging for medical check-up, from January 2010 to December 2011. The inclusion criteria of this study were as follows: (1) premenopausal women, (2) sexually active women (sexual intercourse ≥1 per month), and (3) who completed the questionnaires on female sexual function and the smoking status. The institutional review board of Seoul National University Hospital approved the study (no. 1102-041-351).
The subjects completed the questionnaire regarding their medical/surgical history and socio-demographic characteristics: alcohol consumption, academic career status, and financial status. Alcohol consumption status was divided into two subgroups as never (never drank before) or former (had drank in the past but not currently drinking) and current (drinking at present). The highest level of educational attainment of each individual was regarded as the academic career status and the individual's monthly household income as the financial status. Household income was collected in Korean won.
The female sexual function was compared according to the smoking status, and the smoking status was categorized into two groups: never (never smoked before) or former smokers (had smoked in the past but not currently smoking) and current smokers (smoking at present). The smoking status was determined according to the response to the questionnaire. In current smokers, the sexual function was also evaluated according to the cumulative smoking dose and nicotine dependency. The cumulative smoking dose was calculated in pack-years by multiplying the number of packs smoked per day by the number of years of smoking. Nicotine dependence was assessed using Heaviness Smoking Index (HSI, range 0-6),which is calculated by the sum of two categorical score: number of cigarettes smoked per day (score of 0, 1-10 cigarettes; score of 1, 11-20 cigarettes; score of 2, 21-30 cigarettes; score of 3, >31 cigarettes) and time to first cigarette (score of 0, >60 minutes; score of 1, 31-60 minutes; score of 2, 6-30 minutes; score of 3, ≤5 minutes) [15]. The level of nicotine dependency was divided into two groups based on HSI score: low (HSI score of 0-1) and moderate to high (HSI score of 2-3 and 4-6) dependency [1617].
Female sexual function was assessed using Korean version of female sexual function index (FSFI, range 2-36) [18]. A well-structured questionnaire, FSFI is comprised of 19 items, in which sexual function or problems are evaluated. Sexual function or problem is categorized into 6 domains (desire, arousal, lubrication, orgasm, satisfaction, and pain) and the subjects are allowed to answer in the base of sexual activity within 4 weeks. In each domain, the score ranges from 1 to 5 or 0 to 5, and the sum of score is multiplied by the domain factor (0.3-0.6) to obtain the total score. FSD was defined as the total FSFI score of 26.55 or less [19].
The proportions were compared with Fisher's exact test or chi-square test, and the comparison of continuous variables between each groups were performed with Mann-Whitney U-test. Logistic regression was conducted for multivariate analysis. The results were analyzed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). A P-value <0.05 was considered to be significant.
During this study period, 1,800 sexually active women were approached, and among them, 1,571 (87%) answered the questionnaire. After excluding postmenopausal women or cases with prior hysterectomy, a total of 900 (57%) premenopausal women were enrolled in the analysis. Cases were divided into two groups according to the smoking status: 838 cases of never/former smokers (93.1%, 799 cases of never smokers and 39 cases with former smokers) and 62 cases of current smokers (6.9%).
Table 1 demonstrates the demographics and characteristics of study population according to the smoking status. The median age and the frequency of married or multiparous women were significantly lower in current smokers compared to those of never/former smokers. However, the frequency of current alcohol consumer was significantly higher in cases of current smokers than those of never/former smokers. The median body mass index, the frequency of diabetes and hypertension, and the level of education or income were not different between the two groups.
Among the total study population, 496 (55.1%) women were diagnosed with FSD. Significantly higher frequency of current smokers was observed in cases with FSD than in normal cases (frequency of current smokers: 8.9% in women with FSD and 4.5% in normal women, P=0.011). Table 2 compared the frequency of FSD and total FSFI score between never/former smokers and current smokers. The frequency of FSD was significantly higher in current smokers than in never/former smokers (71.0% in current smokers and 53.9% in never/former smokers, P<0.05), and cases of current smokers had higher frequency of sexual difficulties related to the arousal, lubrication, orgasm, and sexual pain domains than those of never/former smokers. In addition, the total FSFI score was significantly lower in current smokers than in never/former smokers (median total FSFI score [interquartile range]: 24.6 [20.4-27.0] vs. 26.1 [22.8-29.1] in current-smokers vs. never/former smokers, P<0.005), and cases of current smokers also had significantly lower scores in arousal, lubrication, orgasm, and satisfaction domains than those of never/former smokers. This relationship between smoking status and the frequency of FSD remained significant after adjustment for confounding variables (Table 3).
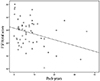
Among 62 current smokers, 53 subjects (85%) completed the questionnaire regarding the number of cigarettes smoked per day and age at first cigarette. Fig. 1 shows the correlation between the cumulative smoking dose (pack-years) and the total FSFI score in current smokers. The cumulative smoking dose (pack-years) and the total FSFI score had a dose-response relationship in which higher cumulative smoking dose was related to the lower total FSFI score (r=-0.278, P<0.05). This negative correlation remained significant after adjustment for confounding variables. The number of packs smoked per day was also associated with the total FSFI score (r=-0.293, P<0.05) and this relationship remained significant after adjustment, whereas the number of years of smoking was not significantly correlated with the total FSFI score (r=-0.153, P=0.260).
Table 4 shows the frequency of FSD and the total FSFI score according to the nicotine dependence in current smokers. The frequency of FSD was higher in moderately to heavily nicotine dependent smokers than in lightly dependent smokers, but was not statistically significant (58% in light dependence and 78% in moderate/heavy dependence P=0.148). However, the frequencies of sexual difficulty in desire and arousal domains were significantly higher in cases of moderate to heavy dependence than in those of light dependence. The total FSFI score of moderately to heavily nicotine dependent smokers was significantly lower than that of lightly nicotine dependent smokers (median total FSFI score [interquartile range]: 21.6 [19.9-26.5] vs. 25.7 [23.9-29.0] in moderate to heavy dependence vs. light dependence, P<0.05), and the cases of moderate to heavy dependence also had lower scores in desire, arousal and pain domains than those of light dependence.
The principal findings of the current study were (1) smoking was an independent risk factor of FSD; (2) there was a dose-related association between smoking and female sexual function in current smokers; (3) nicotine dependence was also correlated with sexual function in current smokers.
Until now, there is a controversy on the association between smoking and FSD. In addition to age, menopausal status, and marital status, smoking is an independent risk factor for FSD in several studies [142021]. However, other studies have not found that association [4612132223]. In the current study, smoking was an independent risk factor for FSD in Korean premenopausal women, and dose-response association was present between cumulative smoking dose and FSD in current smokers. This is an important issue as smoking can lead to impairment of sexual function, which create a major impact on quality of life by damaging self-esteem and interpersonal relationship [324]. Thus, this information could be used in counseling for smoking cessation. Moreover, in this study, nicotine dependency was associated with the risk of FSD in current smokers. This finding was consistent with the report of Harte and Meston [25] who examined the effect of acute nicotine administration on sexual arousal in 20 nonsmoking women, and addressed that nicotine significantly decreased physiologic sexual arousal. Diehl et al. [26] also reported the association between FSD and substance-related disorders, including nicotine dependency.
The current findings can be interpreted according to various mechanisms proposed. First, it can be postulated that smoking itself can inhibit ovarian function and has an anti-estrogenic effect by increasing formation of catechol estrogen metabolites [27]. Estrogen level is an important factor affecting female sexual function, and it is well known that low estrogen level is related to higher prevalence of FSD [28]. In addition, genital vascularization is estrogen sensitive [29]. Anti-estrogenic effect of cigarette smoking results in decreased blood flow into female genital tract [27]. Resistance of uterine, clitoral and labium minus arteries was reported to be significantly higher in smoking women [29]. Pulsatility index of dorsal clitoral artery was inversely correlated to orgasm frequency, and the incidence of vaginal orgasm was significantly lower in heavy smokers than in never smokers [29]. Second, genital engorgement and vascularization may be reduced because of the proatherogenic and vasoconstrictive effect of cigarette smoke components, resulting in stiffness of genital vessel in long term smokers. This could be an explanation for dose-response relationship between smoking and FSD. Decreased nitric oxide level, enhanced b-adrenergic receptor formation and increased anticholinergic effect due to nicotine and/or other cigarette constituents are also responsible for the impaired genital blood flow, which may result in decrease of vaginal lubrication and frequency of sexual intercourse [29]. This adverse effect of nicotine on sexual function corresponds to the result of this study.
There were several limitations of this study. First, the study population consisted of women who visited health promotion center for routine health check-up, and this population may not represent the general premenopausal women. Second, there can be response and social desirability bias due to the utilization of FSFI and questionnaire to assess smoking habits. Third, social factors such as partner factors and sex-related distress could influence sexual function, and these were not considered in this study. Further studies are required for validation including those factors.
In conclusion, current smoking was an independent risk factor for FSD. And cumulative smoking dose and nicotine dependency were associated with higher risk of FSD in Korean premenopausal women.
Figures and Tables
Fig. 1
The relationship between cumulative smoking dose and female sexual function index (FSFI) score among current smokers (r=-0.278, P<0.05).
References
1. Basson R, Berman J, Burnett A, Derogatis L, Ferguson D, Fourcroy J, et al. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol. 2000; 163:888–893.
2. Nappi R, Salonia A, Traish AM, van Lunsen RH, Vardi Y, Kodiglu A, et al. Clinical biologic pathophysiologies of women's sexual dysfunction. J Sex Med. 2005; 2:4–25.
3. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999; 281:537–544.
4. Song SH, Jeon H, Kim SW, Paick JS, Son H. The prevalence and risk factors of female sexual dysfunction in young Korean women: an internet-based survey. J Sex Med. 2008; 5:1694–1701.
5. Hong H, Lee HJ, Kim SM, Jeon MJ, Shin DW, Choi HC, et al. Subclinical hypothyroidism is not a risk factor for female sexual dysfunction in Korean middle-aged women. Thyroid. 2015; [Epub]. DOI: 10.1089/thy.2015.0015.
6. Moreira ED Jr, Kim SC, Glasser D, Gingell C. Sexual activity, prevalence of sexual problems, and associated help-seeking patterns in men and women aged 40-80 years in Korea: data from the Global Study of Sexual Attitudes and Behaviors (GSSAB). J Sex Med. 2006; 3:201–211.
7. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004; 43:1731–1737.
8. Tengs TO, Osgood ND. The link between smoking and impotence: two decades of evidence. Prev Med. 2001; 32:447–452.
9. Mannino DM, Klevens RM, Flanders WD. Cigarette smoking: an independent risk factor for impotence? Am J Epidemiol. 1994; 140:1003–1008.
10. Cao S, Gan Y, Dong X, Liu J, Lu Z. Association of quantity and duration of smoking with erectile dysfunction: a dose-response meta-analysis. J Sex Med. 2014; 11:2376–2384.
11. Polsky JY, Aronson KJ, Heaton JP, Adams MA. Smoking and other lifestyle factors in relation to erectile dysfunction. BJU Int. 2005; 96:1355–1359.
12. Sidi H, Puteh SE, Abdullah N, Midin M. The prevalence of sexual dysfunction and potential risk factors that may impair sexual function in Malaysian women. J Sex Med. 2007; 4:311–321.
13. Cayan S, Akbay E, Bozlu M, Canpolat B, Acar D, Ulusoy E. The prevalence of female sexual dysfunction and potential risk factors that may impair sexual function in Turkish women. Urol Int. 2004; 72:52–57.
14. Oksuz E, Malhan S. Prevalence and risk factors for female sexual dysfunction in Turkish women. J Urol. 2006; 175:654–658.
15. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989; 84:791–799.
16. Cooper J, Borland R, Yong HH, McNeill A, Murray RL, O'Connor RJ, et al. To what extent do smokers make spontaneous quit attempts and what are the implications for smoking cessation maintenance? Findings from the International Tobacco Control Four country survey. Nicotine Tob Res. 2010; 12:Suppl. S51–S57.
17. Borland R, Yong HH, Balmford J, Cooper J, Cummings KM, O'Connor RJ, et al. Motivational factors predict quit attempts but not maintenance of smoking cessation: findings from the International Tobacco Control Four country project. Nicotine Tob Res. 2010; 12:Suppl. S4–S11.
18. Kim HY, So HS, Park KS, Jeong SJ, Lee JY, Ryu SB. Development of the Korean-version of Female Sexual Function Index (FSFI). Korean J Androl. 2002; 20:50–56.
19. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005; 31:1–20.
20. Aslan E, Beji NK, Gungor I, Kadioglu A, Dikencik BK. Prevalence and risk factors for low sexual function in women: a study of 1,009 women in an outpatient clinic of a university hospital in Istanbul. J Sex Med. 2008; 5:2044–2052.
21. Bagherzadeh R, Zahmatkeshan N, Gharibi T, Akaberian S, Mirzaei K, Kamali F, et al. Prevalence of female sexual dysfunction and related factors for under treatment in Bushehrian women of Iran. Sex Disabil. 2010; 28:39–49.
22. Jaafarpour M, Khani A, Khajavikhan J, Suhrabi Z. Female sexual dysfunction: prevalence and risk factors. J Clin Diagn Res. 2013; 7:2877–2880.
23. Safarinejad MR. Female sexual dysfunction in a population-based study in Iran: prevalence and associated risk factors. Int J Impot Res. 2006; 18:382–395.
24. Anastasiadis AG, Davis AR, Ghafar MA, Burchardt M, Shabsigh R. The epidemiology and definition of female sexual disorders. World J Urol. 2002; 20:74–78.
25. Harte CB, Meston CM. The inhibitory effects of nicotine on physiological sexual arousal in nonsmoking women: results from a randomized, double-blind, placebo-controlled, cross-over trial. J Sex Med. 2008; 5:1184–1197.
26. Diehl A, Silva RL, Laranjeira R. Female sexual dysfunction in patients with substance-related disorders. Clinics (Sao Paulo). 2013; 68:205–212.
27. Tziomalos K, Charsoulis F. Endocrine effects of tobacco smoking. Clin Endocrinol (Oxf). 2004; 61:664–674.
28. Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002; 77:Suppl 4. S42–S48.
29. Battaglia C, Battaglia B, Mancini F, Persico N, Nappi RE, Paradisi R, et al. Cigarette smoking decreases the genital vascularization in young healthy, eumenorrheic women. J Sex Med. 2011; 8:1717–1725.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download