Abstract
Objective
Noninvasive prenatal detection of trisomy 21 (T21) has been achieved by measuring the ratio of two alleles of a single nucleotide polymorphism in circulating placenta specific 4 (PLAC4) mRNA in maternal plasma with a few assays in recent years. Our research is to explore the variations of PLAC4 mRNA expression level in maternal plasma with normal pregnancies in second trimester, which can provide pregnant women deeper insights with suitable detection period for the non-invasive prenatal detection of T21.
Methods
We measured a serial plasma PLAC4 mRNA concentrations weekly from the same 25 singleton normal pregnant women. We recruited maternal plasma samples from 45 singleton pregnant women, comprising of 25 euploid pregnancies (control group; range, 17 to 21 weeks) and 20 T21 pregnancies (T21 group; range, 19 to 24 weeks). With the application of reverse transcription polymerase chain reaction, we achieved an insight of PLAC4 mRNA expression levels in maternal plasma during second trimester with euploid pregnancies.
Results
Among the control group, the levels of PLAC4 mRNA expression in the gestation of 17 to 18 weeks were significantly less than those in the gestation of 18 to 21 weeks (P<0.05). The average PLAC4 mRNA concentration of the normal pregnant women was not higher than that of the T21 group (P>0.05).
Current invasive prenatal diagnostic methods for fetal chromosomal aneuploidies, such as amniocentesis and chorionic villus sampling, induce a potential risk to a fetal [1]. Noninvasive screening methods for sonographic and maternal serum biochemical markers have been developed [234], but these markers cannot distinguish the core chromosomal abnormality and are not diagnostic actually. Improvement of methods for investigation of global transcriptional alterations in human disease is now enabling reproducible and consistent selection of genes that best differentiate samples originating from disease-affected or healthy individuals [5].
In recent years, researches proved the feasibility of cell-free DNA testing and integrating the RNA-single nucleotide polymorphism (SNP) approach and circulating mRNA-quantification approach for detecting trisomy 21 (T21) fetuses with PLAC4 mRNA molecules in maternal plasma by mass spectrometry and digital polymerase chain reaction (PCR) methods [67]. For samples with heterozygous SNPs on PLAC4 mRNA, our previous study has confirmed that SNaPshot mini-sequencing assay together with touchdown PCR can be successfully applied for noninvasive prenatal detection of T21 [8]. Nevertheless, the RNA-SNP approach cannot be applied for homozygous fetuses. Besides the RNA-SNP approach, the fetal chromosome 21 dosage can potentially be assessed by measuring the total concentration of PLAC4 mRNA in maternal plasma. It has previously been reported that there was a trend toward an increased PLAC4 mRNA concentration in the maternal plasma of T21 pregnancies, but the increase did not reach statistical significance [9]. While other researchers found that the differences between the euploid group and the T21 one were statistically significant [7]. Based on Lo et al. [9] and Liu et al. [10]'s research, we have learned the clearance of PLAC4 mRNA from maternal plasma within 24 hours after delivery, thus showing its specificity to pregnancy. Besides this, Lo et al. [9]'s study also indicated that there were significant differences in plasma PLAC4 mRNA concentrations between the first trimester and second trimester group as well as between the first trimester and third trimester one, which implied PLAC4 mRNA expression level in maternal plasma during first-trimester was lower than those during the second and the third trimester.
Noninvasive prenatal diagnosis of chromosome aneuploidies (for example, T21) has been achieved by measuring the ratio of two alleles of a SNP in circulating placental PLAC4 mRNA in maternal plasma with Multiple-SNaPshot assay in our previous study. Thus, our study aimed to quantification of PLAC4 mRNA by using reverse transcription real time PCR during different gestational weeks in second trimester, so as to provide deeper insights with suitable and accurate detection period for the noninvasive prenatal detection of T21.
We recruited, with informed consent, a series of pregnant women (2013 to 2014) at the Wuxi Maternal and Child Health Hospital in Jiangsu Province, China. Pregnant women attending their first routine visits in the hospital were offered screening for chromosomal abnormalities, which involved measurement of nuchal translucency and measurement of free β human chorionic gonadotropin in maternal serum. For the identified high-risk women, amniocentesis was offered. In this study, only women with singleton pregnancies were recruited. Samples were prepared from 25 euploid pregnancies and 20 T21 pregnancies. The serial plasma PLAC4 mRNA concentrations were measured weekly from same pregnant women (25 euploid pregnancies). We collected 5 mL of maternal peripheral blood into ethylene diamine tetraacetie acid containing tubes immediately before the procedure. Karyotyping was performed to ascertain the fetal chromosomal status. Our research protocol was approved by the ethical committee (Wuxi Maternal and Child Health Hospital Affiliated Nanjing Medical University).
We stored the unprocessed blood samples at 4℃ upon collection and harvested plasma from the blood samples within 6 hours [11]. The blood samples were centrifuged at 1,600 g for 10 minutes at 4℃. The plasma portion was transferred into clean polypropylene tubes and recentrifuged at 16,000 g for 10 minutes at 4℃. We added 0.75 mL of TRIzol LS reagent (Invitrogen, Carlsbad, CA, USA) and an exogenous cell-mirco-39 RNA (cel-mir-39, with concentration of 10-6 µM) of known quantity per 0.25 mL of plasma before storage at -80℃. Finally, the plasma samples were shipped on dry ice to the laboratory for molecular analysis. All experimental procedures were carried out by a research assistant who had no knowledge of the fetal karyotypes. We extracted RNA from 1 mL of plasma-TRIzol LS mixture prepared as described above according to the manufacturer's protocol for double. We eluted the RNA with 15 µL of RNase-free water, and which was all used in reverse-transcription.
Quantification of RNA isolated from dilute samples such as plasma/serum can be very difficult [12]. Even when using a carrier to enhance the isolation efficiency, attempts to measure RNA concentration by standard spectrophotometry often fail due to the detection limit of this method. As a consequence of these difficulties in quantifying the RNA concentration of dilute solutions, it is in many cases not possible to use equal amounts of template RNA for each sample in the subsequent reverse transcription step. Because large amounts of sample are not always available, often the only alternative is to use equal input volume of RNA. The most commonly used approach here is the use of an exogenous micro RNA (cel-mir-39 in our study, used as endogenous housekeeping control) of known quantity [13]. We then reverse-transcribed the total RNA into cDNA, according to the manufacturer's recommendations of reverse transcriptase (Toyobo, Tokyo, Japan). Simultaneously, we reverse-transcribed endogenous reference cel-mir-39 RNA into cDNA in a same reaction tube, the primer sequences of which: 5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAAGCT-3'. All the primers were synthesized by Biotech Co. (Shanghai, China). The RNA samples were pre-heated before reaction, and then put on ice immediately. The reaction was set up in a reaction volume of 20 µL including 2 µL 5×transfer mix, 1 µL RT enzyme mix, 2 µL primer mix (with final primer concentration of 1 µM) and all the 15 µL pre-heated RNA we extracted. The reaction conditions: 37℃, 45 minutes, 95℃, 5 minutes. A final cDNA volume of approximately of 20 µL was obtained, which was stored at -20℃.
The cDNA samples were then used for real-time PCR measurement with specific primers. The primers for SNP Rs8130833 and cel-mir-39 were designed using Primer 3 software (Table 1). The primers were synthesized by Biotech Co. In this study, we selected primer with the SNP of Rs8130833 to realize the relative quantification of PLAC4 mRNA. The quantification reaction was set up in a reaction volume of 10 µL including 5 µL 2×SyBr-Green mix, 0.2 µL 50×ROX, 1 µL cDNA, 1 µL primer mix (the final primer concentration was listed in Table 1), The PCR was run for 45 cycles of denaturation at 95℃ for 2 minutes, annealing at 96℃ for 15 seconds and elongation at 60℃ for 45 seconds. After 45 cycles, a melting curve analysis was carried out (65℃ to 95℃) to verify the specificity of amplicons. The quantification reactions were performed on ABI PRISM 9700. To minimize stochastic effects, all amplification reactions were run in triplicates. The threshold cycles (Ct) were calculated automatically using the CFX manager software (Bio-Rad, Hercules, CA, USA). The expression level of gene PLAC4 was calculated using the delta-delta Ct method, as previously described [14], by normalizing Ct values to the extrinsic reference cel-mir-39 RNA. All samples gave only a single peak, indicating a single pure product and no primer/dimer formation. Real-time PCR efficiencies were acquired by amplification of a standardized dilution series of the template cDNA and were determined for each gene as the slope of a linear regression model. PCR efficiency was determined by measuring the Ct to a specific threshold for a serial dilution of cDNA. The amplification efficiencies in our study were displayed between 98% to 105%. Correlation coefficients (R2) ranged from 0.98 to 1.0, indicating that all of the primer pairs are highly specific and efficient in polymerase chain reaction amplification.
The relative level of expression (Q) for PLAC4 mRNA was calculated based on the formula Q=2-ΔΔCt. Each Ct value represents the average of three replicates. ΔCt=Ctsamples-Ctcel-mir-39. ΔΔCt=ΔCt (treated)-ΔCt (untreated) [15]. In our study there were no treated samples, so we use ΔCt value to represent relative expression level of PLAC4 mRNA. Statistical analysis was performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as means±standard deviation. Student's t-test was used in the comparison of mean between any two groups. P-values less than 0.05 were considered statistically significant.
Ct value represents the number of cycles that the PCR product appear to effectively increase [16]. By calculating Ct value, we can compare the relative mRNA abundance of each sample of the PLAC4 gene. The higher the Ct value, the lower the mRNA level of the gene; Conversely, the lower the Ct value, the higher the mRNA level of the gene. In our study placental PLAC4 mRNA could be detectable in maternal plasma and showed a certain expression level in different gestational weeks. Among the control group (with normal euploidy pregnancies), the levels of PLAC4 mRNA expression in the gestation of 17 to 18 weeks were significantly less than those of the other three gestations (18 to 19 weeks, 19 to 20 weeks, 20 to 21 weeks; P<0.05) (Table 2). But there was no significant difference between any two of the gestations ranged from 18 to 21 gestational weeks (P>0.05) (Fig. 1).
There was no significant difference in plasma PLAC4 mRNA concentration between the control group and the T21 group (P>0.05) (Fig. 2).
PLAC4 mRNA is transcribed from chromosome 21 and expressed by the placenta in maternal plasma. PLAC4 mRNA in maternal plasma was fetal derived and cleared after delivery. The allelic ratios in maternal plasma correlated with those in the placenta. Based on Tsui et al. [17] and Kido et al. [18]'s researches, we had previously acquired the identification that placental mRNA would be detectable in maternal and showed the highest absolute level of expression in the placenta together with the largest relative difference in expression levels between the placenta and maternal blood cells. Researchers then confirmed that PLAC4 mRNA could be detected in the plasma of women in all three trimesters of pregnancy, but not in the plasma of nonpregnant individuals. As PLAC4 mRNA expression level in maternal plasma during first trimester was lower than those during the second and the third trimester [9], we collected a series of maternal plasma samples from same singleton pregnant women in second trimester with different gestational age (range, 17 to 21 weeks).
Our study on the concentrations of PLAC4 mRNA suggested that plasma PLAC4 mRNA could be detectable in maternal plasma, which was in accordance with that of Lo et al. [9]'s research. Besides than those of 17 to 18 weeks our studies showed a higher level of expression in the gestation of 18 to 21 weeks than those of than those of 17 to 18 weeks among euploid pregnant women, which can provide pregnant women a deeper insight with suitable detection period (18 to 21 weeks) for the noninvasive prenatal detection of T21. Subsequently, the comparison of PLAC4 mRNA expression levels between the two groups were inspected. And the analyses show that there is no significant difference in plasma PLAC4 mRNA expression level between the control group (comprised of 25 euploid pregnancies) and the T21group (comprised of 20 T21 pregnancies). Our results displays a different viewpoint about what is mentioned above [7]. One of the possible reasons was probably that there were individual differences in recruited samples with pregnant women. On the other hand, it could be correlated with the heterogeneity of cells derived from different fetal tissues [19]. Also it maybe because of dysregulation of genome-wide recombination in oocytes with nondisjoined chromosomes 21, which were based on Middlebrooks et al. [20]' recent study. Reports on transcriptomic analyses of other fetal tissues [212223], including cerebellum, heart or fibroblasts, demonstrated that sets of over-expressed and under-expressed genes differ across different cell types. Volk et al. [19] have detected altered levels of mRNAs transcribed from 964 unique genes, with 9 chromosome 21 (HSA21) genes showing significant upregulation in T21 amniocytes, which will predict diagnostic power for discrimination of T21 cases from controls. In their research, some other HSA21 genes didn't show an apparently increased tendency towards differential expression in T21 cases, with consistent in our observation.
We also recognize some limitations of our study. Firstly, the range of gestational age we studied were not wide enough. However, we believe that by increasing a larger width of gestational ages of samples with pregnant women, the insight we supply will be better. Secondly, as plasma are low RNA concentration samples, raising the possibility that preferential isolation of a subset of micro RNA might occur too when non-column purification is used. In addition, data from Michaela's studies have shown that TRIzol LS purification results in higher levels of total RNA recovered from plasma [24].
Among the transcriptomic studies only a few investigated transcriptomic alterations in prenatal T21 samples, including chorionic villus, amniotic fluid cell-free mRNA or amniocytes [2526272829]. The heterogeneity of cells derived from different fetal tissues increases not only the biological variability, but also the possibility of identifying an expression biomarker that could discriminate between a T21 and a normal euploid sample. So what remains to us is to investigate and demonstrate the feasibility of other valuable expression biomarkers to predict high-risk pregnancy status on T21 and other aneuploidy diseases.
In conclusion, we have shown a deeper insight with suitable detection period (18 to 21 weeks) for noninvasive prenatal detection of T21, and we have not found an apparently increased tendency towards PLAC4 mRNA expression among pregnancies with T21 in second trimester.
Figures and Tables
Fig. 1
Relative PLAC4 mRNA expression levels in maternal plasma of different gestational weeks. Graph bars represent the average ΔCt value of the three replicates. The significant differences in ΔCt value in control groups (with euploid pregnancies) between different gestational weeks were calculated using unpaired, two tailed student's test. Ct, threshold cycles. *P<0.05.
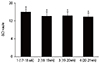
Fig. 2
Relative PLAC4 mRNA expression levels in maternal plasma between the control and trisomy 21 (T21) group. Graph bars represent the average ΔCt value of the three replicates. The significant differences in ΔCt value between the control group (with 25 euploid pregnancies) and the T21 group (with 20 T21 pregnancies) were calculated using unpaired, two tailed Student's test. Ct, threshold cycles. *P>0.05.

Table 1
Polymerase chain reaction primers for SNP, extrinsic reference RNA and concentrations of primers

Acknowledgments
This work was supported by the Maternal and Child Health important project of Jiangsu Province (F201315); Wuxi hospital management center medical technology development fund (CSEYIN1109); and the fundamental project of Nanjing Medical University. Wuxi hospital management center medical fund(YGZXM1510).
We thank Dr D. R. Lu (Fu dan University, Fu Dan-VARI Genetic Epidemiology Center and MOE Key Laboratory of Contemporary Anthropolog) for their cooperation and encouragement.
References
1. Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. 2003; CD003252.
2. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005; 353:2001–2011.
3. Wright D, Syngelaki A, Bradbury I, Akolekar R, Nicolaides KH. First-trimester screening for trisomies 21, 18 and 13 by ultrasound and biochemical testing. Fetal Diagn Ther. 2014; 35:118–126.
4. Breathnach FM, Malone FD. Screening for aneuploidy in first and second trimesters: is there an optimal paradigm? Curr Opin Obstet Gynecol. 2007; 19:176–182.
5. Shi L, Campbell G, Jones WD, Campagne F, Wen Z, Walker SJ, et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat Biotechnol. 2010; 28:827–838.
6. Nicolaides KH, Syngelaki A, Poon LC, Gil MM, Wright D. First-trimester contingent screening for trisomies 21, 18 and 13 by biomarkers and maternal blood cell-free DNA testing. Fetal Diagn Ther. 2014; 35:185–192.
7. Tsui NB, Akolekar R, Chiu RW, Chow KC, Leung TY, Lau TK, et al. Synergy of total PLAC4 RNA concentration and measurement of the RNA single-nucleotide polymorphism allelic ratio for the noninvasive prenatal detection of trisomy 21. Clin Chem. 2010; 56:73–81.
8. Yang L, Sun H, Chen D, Lu M, Wang J, Xu F, et al. Application of multiplex SNaPshot assay in measurement of PLAC4 RNA-SNP allelic ratio for noninvasive prenatal detection of trisomy 21. Prenat Diagn. 2014; 34:139–144.
9. Lo YM, Tsui NB, Chiu RW, Lau TK, Leung TN, Heung MM, et al. Plasma placental RNA allelic ratio permits noninvasive prenatal chromosomal aneuploidy detection. Nat Med. 2007; 13:218–223.
10. Liu HY, Yang LL, Li F, Yang HJ, Liu SM, Zhang JB. Detection and clinical significance of fetal specific mRNA from peripheral maternal blood. Zhonghua Fu Chan Ke Za Zhi. 2011; 46:655–657.
11. Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002; 48:1647–1653.
12. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010; 50:298–301.
13. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008; 105:10513–10518.
14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
15. Li QF, Jiang MY, Yu HX, SW Xin, MH Gu, QQ Liu. Selection of internal reference genes for quantitative RT-PCR analysis of total RNA from endosperm of rice (Oryza sativa L.). J Yangzhou Univ. 2008; 29:61–66.
16. Scharlaken B, de Graaf DC, Goossens K, Brunain M, Peelman LJ, Jacobs FJ. Reference gene selection for insect expression studies using quantitative real-time PCR: the head of the honeybee, Apis mellifera, after a bacterial challenge. J Insect Sci. 2008; 8:33.
17. Tsui NB, Chim SS, Chiu RW, Lau TK, Ng EK, Leung TN, et al. Systematic micro-array based identification of placental mRNA in maternal plasma: towards non-invasive prenatal gene expression profiling. J Med Genet. 2004; 41:461–467.
18. Kido S, Sakuragi N, Bronner MP, Sayegh R, Berger R, Patterson D, et al. D21S418E identifies a cAMP-regulated gene located on chromosome 21q22.3 that is expressed in placental syncytiotrophoblast and choriocarcinoma cells. Genomics. 1993; 17:256–259.
19. Volk M, Maver A, Lovrecic L, Juvan P, Peterlin B. Expression signature as a biomarker for prenatal diagnosis of trisomy 21. PLoS One. 2013; 8:e74184.
20. Middlebrooks CD, Mukhopadhyay N, Tinker SW, Allen EG, Bean LJ, Begum F, et al. Evidence for dysregulation of genome-wide recombination in oocytes with nondisjoined chromosomes 21. Hum Mol Genet. 2014; 23:408–417.
21. Mao R, Wang X, Spitznagel EL Jr, Frelin LP, Ting JC, Ding H, et al. Primary and secondary transcriptional effects in the developing human Down syndrome brain and heart. Genome Biol. 2005; 6:R107.
22. Li CM, Guo M, Salas M, Schupf N, Silverman W, Zigman WB, et al. Cell type-specific over-expression of chromosome 21 genes in fibroblasts and fetal hearts with trisomy 21. BMC Med Genet. 2006; 7:24.
23. Conti A, Fabbrini F, D'Agostino P, Negri R, Greco D, Genesio R, et al. Altered expression of mitochondrial and extracellular matrix genes in the heart of human fetuses with chromosome 21 trisomy. BMC Genomics. 2007; 8:268.
24. Kirschner MB, van Zandwijk N, Reid G. Cell-free microRNAs: potential biomarkers in need of standardized reporting. Front Genet. 2013; 4:56.
25. Altug-Teber O, Bonin M, Walter M, Mau-Holzmann UA, Dufke A, Stappert H, et al. Specific transcriptional changes in human fetuses with autosomal trisomies. Cytogenet Genome Res. 2007; 119:171–184.
26. FitzPatrick DR, Ramsay J, McGill NI, Shade M, Carothers AD, Hastie ND. Transcriptome analysis of human autosomal trisomy. Hum Mol Genet. 2002; 11:3249–3256.
27. Rozovski U, Jonish-Grossman A, Bar-Shira A, Ochshorn Y, Goldstein M, Yaron Y. Genome-wide expression analysis of cultured trophoblast with trisomy 21 karyotype. Hum Reprod. 2007; 22:2538–2545.
28. Chou CY, Liu LY, Chen CY, Tsai CH, Hwa HL, Chang LY, et al. Gene expression variation increase in trisomy 21 tissues. Mamm Genome. 2008; 19:398–405.
29. Slonim DK, Koide K, Johnson KL, Tantravahi U, Cowan JM, Jarrah Z, et al. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc Natl Acad Sci U S A. 2009; 106:9425–9429.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download