Abstract
Peritoneal origin serous papillary carcinoma is an uncommon primary malignancy occurring in the abdominal or pelvic peritoneum lining. It is characterized by peritoneal carcinomatosis with massive ascites, uninvolved or minimally involved ovary, and is histologically indistinguishable from ovarian serous tumors. Better recognition of this phenomenon in recent years has contributed to an increasing diagnostic frequency. We describe a rare case of peritoneal origin serous papillary carcinoma with unusual clinical presentations involving a solitary primary tumor originating from the peritoneal lining of the sigmoid colonal mesentery, without pelvic lymph node involvement or distant metastasis. Because of the location and morphological similarity, it was misdiagnosed as an ovarian malignancy. We aim to assist in the diagnosis of this disease with the following case report, thereby improving the management of patients with this condition.
Peritoneal serous papillary carcinoma (PSPC) is a rare primary malignancy that arises in the peritoneal lining of the abdomen and pelvis without discriminative primary tumor site. It is considered to originate from embryonic nests of müllerian cells in the peritoneum [1]. It is similar to papillary serous ovarian carcinoma with respect to clinical presentation, histological appearance, pattern of spread, treatment and prognosis. Furthermore, it is nearly impossible to distinguish PSPC from papillary serous ovarian carcinoma based on clinical and imaging findings alone. Particularly, PSPCs are characterized by either peritoneal carcinomatosis with no or minimal involvement of the ovaries and no identifiable primary tumor [2]. In almost all cases of PSPC, extensive intraperitoneal spread is detected at the time of diagnosis, even in the absence of evidence of a primary ovarian tumor. Interestingly, however, we have encountered a case of PSPC that presented as localized pelvic masses without peritoneal dissemination, pelvic lymph node involvement or distant metastasis. It is not only rare in the world, but also there is even no one reported case in Korea. The expression of the case was very unusual, for the uniqueness of it, we decided to report. Although rare, such unusual presentations of PSPC might make determining a definitive diagnosis even more difficult.
A 73-year-old woman arrived at the gynecologic department complaining of palpable abdominal mass. Because of voiding difficulty for several months previously, she visited the local urologist. In ultrasound, an abnormal finding was detected, and she was recommended to undergo gynecologic examination. At the local gynecologic hospital, she underwent abdomino-pelvic computed tomography (CT) and hematologic tests. In those tests, an abnormal CT finding and a high level of serum CA-125 as 719 U/mL (0-35) were detected. And she was transferred to our clinic for surgery. She indicated neither the presence of any past medical problems, involving neither remarkable gynecological issues nor family history. A physical examination revealed a large, solid, non-tender mass in the left lower abdomen; no other masses were detected. A laboratory examination revealed an elevated level of CA-125 of 547 U/mL. The serum level of CA-72-4 was elevated to 12.58 U/mL. CA-19-9, human chorionic gonadotropin, alpha fetoprotein and carcinoembryonic antigen were within the normal ranges. Intravaginal ultrasonography revealed a large, ovoid mixed component mass in the left adnexa (Fig. 1A). According to the local CT reading, the shape of the mass appeared as a borderline or malignant ovarian tumor (Fig. 1B).
Dynamic CT scans were obtained after starting the injection of contrast material. There was a huge cystic mass with a length of 12 cm in the pelvic cavity, with irregular wall enhancement, and a large, ovoid-shaped mass, which displayed attenuation similar to that of blood vessels and was tightly adhered to the colonic mesentery. In the early phase, the mass was strongly and rapidly enhanced. An unremarkable uterus was visualized; however, the bilateral adnexa could not be detected. Based on the hematologic and radiologic findings, a preoperative diagnosis suggested a left ovarian cystic mass with boderline concern. A TORSO Fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT reading was obtained in one hour following 8.2 mCi of F18 FDG by intravenous injection. An approximately 6.3×6.5×5-cm-sized cystic mass was identified, with thick hypermetabolic rim excluding the anterior and right lateral margin in the lower abdomen above uterus. Otherwise, there was no evidence of abnormal increased FDG uptake in the patient's body from skull base to upper thigh (Fig. 1C). In exploratory laparotomy, a fist-sized, necrotized, protruding mass was discovered on the mesenteric aspect of the sigmoid colon. As a result, we attempted to detach the pericolic tissue and the mass, but this was impossible. The uterus and bilateral adnexa were atrophied and free of disease. The omentum, liver and diaphragm were all palpated and showed no remarkable lesions. There were no disseminated nodules in the upper peritoneal cavity. The left periovarian soft tissue had a walnut-sized mass. The cul-de-sac and uterosacral ligamental portions showed some disseminated nodules. No enlarged pelvic, pericolic or para-aortic lymph nodes or other retroperitoneal pathologies were identified. A relatively well-circumscribed, solid cystic component mixed multiple lobulated tumor measuring 12.0×6.5×6.0 cm was located on the peritoneum mesenteric lining of the sigmoid colon. We performed aspiration of its cystic component to enable extirpation. Approximately 300 mL of opaque fluid was aspirated. We performed both salpingo-oophorectomy, partial omentectomy, pelvic lymphadenectomy, and removal of the mesenteric mass. We sought to execute optimal cytoreductive simultaneous surgical staging. But the uterus was fixed tightly by severe adhesion, we could not perform hysterectomy. We suggested a segmental resection of sigmoid colon to a co-operative general surgeon, but he did not agree with our opinion. As a result, we conducted suboptimal cytoreductive surgery. In the frozen section, both ovaries were normal; the mesenteric mass was a carcinoma.
Grossly, the specimen of the mesenteric cystic mass of the sigmoid colon was intraoperatively cystic fluid aspirated. The collapsed cystic mass shows inner yellowish necrotic debris and a cystic wall. Microscopic findings show a fibrotic cystic wall, necrotic debris, and a papillary growing mass, all characteristics of serous papillary carcinoma (Fig. 2A, B). Peritoneal cavity washing cytology was negative for malignancy. The left ovary was atrophied and measured 1.2×0.5×0.5 cm. Adjacent to the left ovary, a yellowish-brown colored solid nodular mass was also identified and measured 3.0×0.5×0.5 cm. Microscopic findings of this solid mass showed solid and papillary growing tumor mass, consistent with papillary serous carcinoma of the ovary (Fig. 2C). Numerous psammoma bodies were also noted (Fig. 2D). The left atrophied ovary was free from tumor. The right ovary measured 1.5×0.6×0.5 cm. Microscopically, the right ovary showed a focus of tumor emboli in lymphatic and tumor implants in paraovarian soft tissue. Immunohistochemical staining of calretinin, carcinoembryonic antigen (CEA), vimentin (VMT), cytokeratins 7 (CK7) was performed. The tumor cells showed positively for CK7 and negative for VMT, calretinin, and CEA (Fig. 2E).
After surgery, the patient recovered without complication. One week after the operation, tumor antigens were rechecked. CA-125 level was 173 U/mL, and CA-72-4 level was 9.22 U/mL. Postoperative day 12 she was discharged. After 1 month from the operative day, the patient had readmission and follow up PET-CT and hematologic tests. The subsequent CA-125 level was 553 U/mL, while the CA-72-4 level was 15.15 U/mL. PET-CT did not detect any other metastatic lesion. The patient was administered 1 cycle of paclitaxcel-carboplatin chemotherapy, and discharged. The patient will be treated by paclitaxcel 238 mg, carboplatin 544 mg every 3 weeks. A follow up of CA-125 level is scheduled every 3 weeks before the patient begins each chemotherapy.
We have described a case of localized PSPC presenting as a solitary colonic mass. The histopathological findings of the tumor were consistent with the diagnosis of serous papillary carcinoma, but not colonic adenocarcinoma. The immunohistochemical results excluded the possibility of the tumor cells being of mesothelial origin. The absence of ovarian disease indicated a primary peritoneal origin. The tumor was localized in the sigmoid colonal mesentery, but without the presence of spreading in the pelvic cavity or distant metastasis. Tumoral infiltration was limited in the mesentery; the colonal lumen was totally intact and the patient had no bowel habit change. The most remarkable aspect of the present case was its unusual pattern of spread. Typically, PSPC gives rise to dissemination on the peritoneal surface and greater omentum in its early phase of growth. Furthermore, it is not uncommon to observe tumor implants on the surface of the liver, diaphragm or mesentery [3]. PSPC is seen only in microinvasive or normal ovaries, and is thought to originate from the peritoneum, but histologically it is difficult to distinguish from epithelial ovarian cancer. At the time of diagnosis of PSPC, other primary cancers, especially in differentiating primary ovarian cancer, should be considered. The diagnostic criteria for primary peritoneal cancer vary; Mills et al. [4] diagnosed only if the ovary represents less than 3 cm in diameter showing no invasion or microinvasion. However, Fromm et al. [5] claimed the maximum diameter of normal ovaries to be less than 4 cm. Mulhollan et al. [6] defined the diameter of ovary to be 3 cm or less and the surface of the ovarian tumor size to be less than 5 mm; into the ovarian parenchymal microinvasion was less than 3 mm. Recently, the Gynecologic Oncology Group recommended a diagnostic criterion of PSPC. It should be histologically similar to papillary serous ovarian cancer, and do not invasive or do not infiltrate the ovary in place. If the case of the ovarian microinvasion, that should not exceed 5×5 mm, and lesions of the peritoneal region larger than other primary ovarian lesions. And it should not be able to find other primary cancer site [7]. There were some unusually diagnosed PSPC cases. One of them was using the Pap test, the psammoma body was found. The other cases were in the course of abdominal surgery for other reasons, the peritoneal cancer was reported to be found [6]. In order to preoperative evaluation for the patients with ovarian cancer, should be performed thorough history taking, and a physical examination, and should include radiographic and laboratory findings. In ultrasonographic tests, even if the ovary finding was normal, on the surface of the peritoneum or bowel, invasive nodules may be seen. The CT scan is known to be helpful to differentiate the disease of the peritoneum, the retroperitoneum, liver, and obstruction of urinary tract. Zissin et al. [8] reported that the most common abdominal CT findings of PSPC including various degrees of ascites, great omental invasion, irregular thickening of the parietal peritoneum, S-colon wall thickening, and chest wall findings including thickening of the diaphragmatic nodules, enlarged heart, and pleural effusion. However, the primary diagnosis of PSPC cannot be accomplished using only radiographic examination, because we cannot discriminate metastatic peritoneal carcinomatosis from peritoneal mesothelioma. The effort to identify the primary lesion of mammary, pancreatic, gastrointestinal, or other systems should be performed, and as a tumor marker, the CA-125 level should also be checked as an important diagnostic tool. Through the paracentesis, a positive predictive value of cytology is reported to be high, but the sensitivity is poorer. PSPC was considered as a differential diagnosis before surgery, but the final diagnosis was made at the time of surgery and as a result of histopathological evaluation [7].The pathogenesis of PSPC has still not been clearly identified. In 1972, Lauchlan [9] noted that, for women, the peritoneum is a portion of the secondary Müllerian organs, and therefore, they argued that the surface epithelium of the ovary or peritoneal mesothelium have the same generative origins, and they hold the pluripotency of Müllerian organs. These versatile characteristics of peritoneum are able to explain the atypical squamous metaplasia of it or the occurence of endometriosis and adenomyosis from the peritoneum. Considering the number of generated patterns, Truong et al. [3] reported that such diseases occurring in the peritoneum and fallopian tube, endometrium are affected by a kind of Müllerian organ involving hormones, as the potential to become an important etiology. In PSPC, but estrogen and progesterone receptor expressions are rather unusual as approximately 38%, and show similar patterns in epithelial ovarian cancer, which show 32% of expressions [7]. Other hypothesis for the pathogenesis is the ovarian germ cells that involved later stages and remaining in the process, easy to cause malignant degeneration [10]. PSPC diagnosis and staging of ovarian cancer are the same as the staging of ovarian cancer surgery, which the International Federation of Gynecology and Obstetrics stagings generally follow [7]. Lele et al [11]. found that in PSPC, the initial metastasis of carcinoma is more severe, and also the larger the residual cancer, the less response to postoperative chemotherapy. They described the need for appropriate initial cytoreductive surgery. In addition, initially using cisplatin with chemotherapy alone showed more than 65% treatment effect. In particular, complex compounds based on cisplatin chemotherapy were more effective than those of monotherapy. Fromm et al. [5] reported, in 74 cases of PSPC, a much higher survival rate (19.5 vs. 31.5 months) when compounds based on cisplatin chemotherapy were used, than when cisplatin monotherapy was performed. Chen and Flam [12] reported on three cases of PSPC, using cisplatin and adriamycin combination chemotherapy: a survival rate of more than five years was shown. In the Gynecologic Oncology Group study, PSPC and primary ovarian cancer were treated with cisplatin (75 mg/m2) and cyclophosphamide (750 mg/m2). No difference in effectiveness and in toxicity was observed between the two groups [13]. Chu et al. [7] also reported in a PSPC case that proper cytoreductive surgery was not perfomed; Taxol therapy raised the survival rate. Eltabbakh et al. [14], in the research on primary care and for prognostic factors in 75 PSPC cases, patient age, surgical staging, activity score, and whether cytoreductive surgery occurred were reported as prognostic factors. They reported that optimal cytoreductive surgery for residual tumors less than 1 cm in size, such as p53 over-expression and estrogen, progesterone receptors and histologic type, had no impact on the survival rate of patients. Conclusively, compared with ovarian cancer, PSPC is a rare tumor which shows incidence of 4.6%, but providing an accurate diagnostic criterion, is more likely to increase the incidence. Therefore, a case with ascites, multiple peritoneal disseminated tumors, and ovary or other primary organs observed in the absence of lesions, should be suspected as primary peritoneal cancer, and measurement of serum CA-125 level, abdomino-pelvic CT scan or magnetic resonance imaging should be performed. Treatment is similar as in epithelial ovarian cancer: cytoreductive surgery including surgical staging on the basis of platinum chemotherapy, which is the normalization of CA-125 after 3months of diagnosis. Whether the prognosis is poor may help to determine the course of treatment [15]. But in order to establish the clinical pathologic features and the prognostic factors of PSPC, additional more prospective studies of patients are needed in the future.
Figures and Tables
Fig. 1
(A) Transvaginal ultrasonography showed a large mixed component mass (arrow pointing) regarded as a left ovarian tumor. (B) Dynamic computed tomography scans were obtained after starting the contrast material injection. There was a large mass (arrow pointing) with attenuation similar to that of blood vessels that tightly adhered to and externally compressed the colonic wall. In the early phase, the mass was strongly and rapidly enhanced. (C) On the TORSO Fluorine-18 fluorodeoxyglucose positron emission tomography and computed tomography, an approximately 6.3×6.5×5-cm-sized cystic mass was identified, with thick hypermetabolic rim excluding anterior and right lateral margin in the lower abdomen above the uterus.
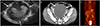
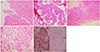
Fig. 2
Histopathological and immunohistochemical findings. (A) The photomicrographic result. It shows a fibrocystic wall and necrotic tissue from the mesenteric mass (H&E, ×10). (B) Photomicrograph from mesenteric mass showing papillary architecture of tumor (H&E, ×20). (C) Left periovarian mass. It is soft tissue from left adjacent to papillary growing mass (H&E, ×10). (D) The photomicrograph result. It shows a multilayered papillary growing tumor. Numerous scattered psammoma bodies were present (H&E, ×10). (E) Immunohistochemically, the tumor cells were positive for only cytokeratins 7 (CK7); others (vimentin [VMT], carcinoembryonic antigen [CEA] and calretinin) were negative.
References
1. Sehgal S, Agarwal R, Goyal P, Singh S, Kumar V, Gupta R. Primary serous carcinoma of peritoneum: a case report. Int J Case Rep Images. 2012; 3:16–20.
2. Eltabbakh GH, Piver MS. Extraovarian primary peritoneal carcinoma. Oncology. 1998; 12:813–819.
3. Truong LD, Maccato ML, Awalt H, Cagle PT, Schwartz MR, Kaplan AL. Serous surface carcinoma of the peritoneum: a clinicopathologic study of 22 cases. Hum Pathol. 1990; 21:99–110.
4. Mills SE, Andersen WA, Fechner RE, Austin MB. Serous surface papillary carcinoma: a clinicopathologic study of 10 cases and comparison with stage III-IV ovarian serous carcinoma. Am J Surg Pathol. 1988; 12:827–834.
5. Fromm GL, Gershenson DM, Silva EG. Papillary serous carcinoma of the peritoneum. Obstet Gynecol. 1990; 75:89–95.
6. Mulhollan TJ, Silva EG, Tornos C, Guerrieri C, Fromm GL, Gershenson D. Ovarian involvement by serous surface papillary carcinoma. Int J Gynecol Pathol. 1994; 13:120–126.
7. Chu CS, Menzin AW, Leonard DG, Rubin SC, Wheeler JE. Primary peritoneal carcinoma: a review of the literature. Obstet Gynecol Surv. 1999; 54:323–335.
8. Zissin R, Hertz M, Shapiro-Feinberg M, Bernheim J, Altaras M, Fishman A. Primary serous papillary carcinoma of the peritoneum: CT findings. Clin Radiol. 2001; 56:740–745.
9. Lauchlan SC. The secondary Müllerian system. Obstet Gynecol Surv. 1972; 27:133–146.
10. Kannerstein M, Churg J, McCaughey WT, Hill DP. Papillary tumors of the peritoneum in women: mesothelioma or papillary carcinoma. Am J Obstet Gynecol. 1977; 127:306–314.
11. Lele SB, Piver MS, Matharu J, Tsukada Y. Peritoneal papillary carcinoma. Gynecol Oncol. 1988; 31:315–320.
12. Chen KT, Flam MS. Peritoneal papillary serous carcinoma with long-term survival. Cancer. 1986; 58:1371–1373.
13. Bloss JD, Brady MF, Liao SY, Rocereto T, Partridge EE, Clarke-Pearson DL, et al. Extraovarian peritoneal serous papillary carcinoma: a phase II trial of cisplatin and cyclophosphamide with comparison to a cohort with papillary serous ovarian carcinoma-a Gynecologic Oncology Group Study. Gynecol Oncol. 2003; 89:148–154.
14. Eltabbakh GH, Werness BA, Piver S, Blumenson LE. Prognostic factors in extraovarian primary peritoneal carcinoma. Gynecol Oncol. 1998; 71:230–239.
15. Barakat RB, Markman M, Tandall ME. Primary peritoneal carcinoma. Principles and practice of gynecologic oncology. 15th ed. Philadelphia(PA): Lippincott Williams & Wilkins;2009. p. 810–813.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download