Abstract
Objective
To analyze the diagnostic profiles and treatment outcomes of patients with thoracic endometriosis at a university hospital.
Methods
A retrospective review of medical records was performed for patients diagnosed with thoracic endometriosis at Gangnam Severance Hospital, Yonsei University College of Medicine, between January 2007 and January 2014.
Results
Fifteen patients (median age, 35 years; range, 23-48 years) were evaluated. Patients presented with catamenial hemoptysis (n=8), or catamenial pneumothorax (n=7). Patients with catamenial pneumothorax were significantly older than those presenting with hemoptysis (P=0.0002). Only 3 patients (20%) had coexisting pelvic endometriosis. All patients underwent chest computed tomography; lesions were shown to predominantly affect the right lung (right lung, n=13, 86.7%; left lung, n=2, 13.3%), and were mainly distributed on the right upper lobe (n=9, 60%). Ten patients underwent video-assisted thoracoscopic surgery, and 1 patient underwent a thoracotomy. Intraoperatively, endometriosis-specific findings were observed in 8/11 patients (72.7%); a further 5/11 patients (45.4%) had histologically detectable endometriosis. Over the follow-up period (mean, 18.4 months; range, 2-65 months) 5/15 patients (33%) had clinical signs of recurrence. Recurrence was not detected in any of the 5 catamenial pneumothorax patients that received adjuvant hormonal therapy after surgery.
Conclusion
The diagnosis and management of thoracic endometriosis requires a multidisciplinary approach, based upon skillful differential diagnosis, and involving careful gynecologic evaluation and assessment of the cyclicity of pulmonary symptoms. Imaging findings are non-specific, though there may be laterality towards the right lung. Since symptom recurrence is more common in those with presenting with pneumothorax, post-operative adjuvant medical therapy is recommended.
Endometriosis is defined as the presence of endometrial tissue outside the uterine cavity. The ectopic (extra-uterine) endometrial tissue is histologically characterized by the presence of endometrial stroma and glands [1]. Endometriosis is considered an enigmatic disease, the exact mechanism of which remains unknown. Consequently, clinical eradication of the disease is very difficult, and recurrence is common [1,2].
While endometriosis is usually confined to the pelvis, it is also known to occur in extra-pelvic organs and tissues. Extra-pelvic endometriosis, although rare (8.9%), may occur in the gastrointestinal tract (32.3%), the urinary tract (5.9%), and other sites (61.8%), which include the lung, the umbilicus, abdominal scars, the liver, the gall bladder, the pancreas, the breasts, and the extremities [3,4,5,6,7].
Thoracic endometriosis is defined as the presence of ectopic endometrial tissue inside the thoracic cavity [8]. It usually presents with pneumothorax, hemothorax, hemoptysis, lung nodules, isolated chest pain or pneumomediastinum; symptoms are synchronized with the menstrual cycle [5].
A pneumothorax occurring between 24 hours before and 72 hours after the onset of menstruation is described as "catamenial." Although this may include primary spontaneous pneumothorax, occurring coincidently during the perimenstrual period, the majority of recurrent episodes of catamenial pneumothorax are caused by thoracic endometriosis. It is encountered in 20% to 30% of women with spontaneous pneumothorax [9].
Catamenial hemoptysis is characterized by cyclic pulmonary hemorrhage, synchronized with menstruation, which is associated with the presence of parenchymal or intra-bronchial endometrial tissue. Despite interest in thoracic endometriosis, the clinical profiles and etiology remain undetermined and obscure. Comprehensive analyses of women with catamenial pneumothorax or hemoptysis in the literature are lacking, likely due to the rarity of these symptoms. In the literature there is very little consensus management plans, although it is known that the recurrence rate is very high with conservative management only.
To better understand this enigmatic disease, we performed a retrospective analysis of 15 patients with thoracic endometriosis, to determine the demographics, clinical presentations, pathological findings, and effectiveness of treatment, within a cohort from a single university hospital.
We performed a retrospective analysis of the medical records of women diagnosed with thoracic endometriosis (presenting with catamenial pneumothorax, hemothorax, hemoptysis, and/or lung nodules) between January 2007 and January 2014, at Gangnam Severance Hospital, Yonsei University College of Medicine.
Inclusion criteria consisted of the following: 1) women of reproductive age, 2) sufficient identifiable clinical information through history taking, physical examination, and imaging studies, 3) diagnosis based on the characteristics of intraoperative findings, 4) diagnosis based on histopathological confirmation, and 5) patients with full gynecological evaluation. Exclusion criteria consisted of the following: 1) presence of other active lung disease/unable to exclude malignancy and 2) loss to follow-up while still symptomatic. A total of 15 patients were appropriate for inclusion in our study. All patients were admitted to our institution for diagnosis or treatment. The study was approved by our institutional review board.
Medical records, including out-patient and in-patient reports and radiology results, were thoroughly reviewed. The follow-up period included the time from diagnosis to the completion of all treatment or indefinitely, until they relapsed. The type and duration of adjuvant hormone treatment, if prescribed, and recurrence of disease were analyzed. All 15 women underwent a full gynecological evaluation after the diagnosis of lung lesions with pelvic ultrasonography and/or computed tomography (CT) scan, before the start of adjuvant therapy; serum CA-125 was also measured in all patients.
The clinical profiles of all patients diagnosed with and treated for thoracic endometriosis at our institution are described in Table 1. A total of 21 patients were retrieved, 15 of which were eligible for inclusion. Of these 15, 8 patients were diagnosed with thoracic endometriosis with hemoptysis as their chief complaint, and 7 patients presented with pneumothorax. The median age was 35 years (range, 23-48 years). All patients displayed some degree of catamenial symptoms, although patterns differed; some patients reported symptoms with every cycle of menstrual bleeding (n=7), whereas others showed only occasional episodes during menstruation (n=8). Patients had experienced between 1 and 7 catamenial episodes before presenting for medical advice. None of the 15 patients were smokers; 1 patient had a history of asthma, and 2 patients presenting with catamenial hemoptysis had histories of empirical tuberculosis medication-use prior to being diagnosed with thoracic endometriosis. Three patients had previously undergone video-assisted thoracoscopic surgery (VATS), undertaken at other centers, following which they had not been diagnosed with thoracic endometriosis. In 1 patient with a history of pneumothorax (P12), the episode occurred on the contralateral side to the presence of endometriotic lesions and the underlying etiology was considered equivocal.
Only 3/15 (20%) patients had coexisting pelvic endometriosis (P1, P9, and P10). Of these, P1 underwent laparoscopy-guided enucleation of an ovarian endometriotic cyst; P9 displayed the typical "ground-glass" echogenicity of an ovarian endometriotic cyst on ultrasonography, but declined surgical removal due to the absence of any symptoms; P10 had a history of total hysterectomy with left salpingo-oophorectomy 6 years previously, and had a histological diagnosis of pelvic endometriosis. Thirteen of the 15 patients had a history of at least 1 pregnancy (gravidity or parity); no patient reported a history of infertility. Two patients (P1 and P9), both with pelvic endometriosis, had elevated serum CA-125.
Preoperative imaging profiles are summarized in Table 2. All patients underwent CT imaging of the chest for diagnosis. Locations of the lesions shown on the CT scan were analyzed; lesions were identified in the right lung (n=13, 86.6%), and the left lung (n=2, 13.4%). The majority of lesions in the right upper lobe (n=9, 60%). No patients had bilateral lesions. In patients presenting with hemoptysis, CT findings were primarily of ground glass opacity, suggesting pulmonary hemorrhage, nodular lesions, and air filled cavities. All the patients presenting with hemoptysis had normal chest radiographs. One patient (P2) in whom the initial thoracic CT scan was unremarkable was subsequently shown to have a focal ground glass opacity lesion in a later CT scan, undertaken during her menstrual period. In those patients presenting with pneumothorax, there were no CT findings suggestive of endometriosis (Table 2).
Management of the study patients is summarized in Table 3. With regard to management, the majority of patients (11/15) underwent surgery, while three out of 15 received hormone treatment only, and one patient received bronchial artery embolization as the initial treatment. Of the patients that presented with catamenial hemoptysis, 4 underwent VATS, 1 (P3) received bronchial artery embolization, and 3 underwent hormone therapy with GnRH agonist as the primary treatment. The various treatment modalities attempted reflect the lack of specific treatment guidelines for catamenial hemoptysis. All 7 catamenial pneumothorax patients received thoracic surgery as the initial mode of treatment; 6 underwent VATS, and 1 underwent a thoracotomy.
Intraoperative findings are summarized in Table 4. Specific intraoperative findings characteristic of endometriosis (red lesions, endometriotic spots) were observed in 9/11 (81%) of the patients who underwent thoracic surgery; 5 (45%) of these patients had endometriosis pathologically confirmed from a thoracic specimen. The dominant intraoperative findings in patients with hemoptysis consisted of pleural endometriotic spots, hemorrhages, bullae, and blebs (Fig. 1A, B). In those with pneumothorax, ≥1 diaphragmatic defects, ranging from 1 to 5 mm were discovered, often coexisting with endometriotic spots in the adjacent tissues (Fig. 1C, D).
Histopathological findings are summarized in Table 3. Specimens from 5/11 (45.4%) patients displayed the hallmark finding of 'endometrial glands and stroma,' in 1 patient the specimen displayed only endometrial stromal cells. Another 2 patients had findings suggestive of endometriosis, including fibrosis without endometriotic glands. Hemosiderin laden macrophages, considered compatible with or suggestive of endometriosis, were present in another 2 patients.
Table 4 summarizes the clinical course and outcomes of study patients. Mean follow-up time for the group was 18.4 months (range, 2-65 months). Of the patients that presented with hemoptysis, 3/4 (75%) patients in the surgical treatment group did not experience recurrence. In contrast, 2/3 (67%) patients experienced recurrence in the hormone-only treatment group; these patients had been treated with dienogest (Visanne, a dosage of 2 mg/day) as maintenance, following 3 cycles of GnRH agonist (leuprolide acetate depot 3.75 mg every 4 weeks) induction.
In the surgical treatment group, 1 patient with coexisting pelvic endometriosis experienced recurrence, and was treated with thoracic re-operation, without subsequent adjuvant medical therapy. Although the recurrence rate appears higher in the hormone treatment group, P6 had only one minor episode during 10 months follow-up, and P7 had a significant reduction in the amount of hemoptysis. The patient (P8) who received bronchial artery embolization and adjuvant hormone therapy with a GnRH agonist was successfully treated, without relapse.
Of the patients that presented with a pneumothorax, there were 3 episodes of recurrence in 2 patients (mean follow-up, 26.5 months; range, 10-65 months); neither had received postoperative adjuvant hormonal therapy. Following surgery, 5/7 patients received adjuvant hormonal therapy with a GnRH agonist; this comprised 2 to 6 cycles of a GnRH agonist, followed by medroxyprogesterone acetate (n=1) or an oral contraceptive (n=4), for 3 months. We recommended 6 cycles of the GnRH agonist, but 2 patients declined further cycles, after either 2 or 4 cycles, because of menopausal symptoms. However, in those who received serial adjuvant hormone therapy with a GnRH agonist, recurrence was not detected.
While various hypotheses have been postulated to explain thoracic endometriosis, none have been confirmed. Hypotheses include coelomic metaplasia of the pelvic or distant tissues into ectopic endometrial tissue; the physiologic hypothesis, where high levels of circulating prostaglandin F2 during menstruation causes vasoconstriction and bronchospasm, with subsequent alveolar rupture and pneumothorax; the migration theory, in which endometrial diaphragmatic implants may result from the migration of endometrial tissue from the uterus to the pelvis, and through the peritoneal fluid to the subdiaphragmatic area; and metastatic or lymphovascular micro-embolization, which suggests a metastatic spread of endometrial cells to the lungs, through the venous or lymphatic vasculature. Finally, the transgenital-transdiaphragmatic passage of air theory suggests that the passage of air through congenital or acquired (secondary to endometriosis) diaphragmatic defects may result in ectopic implants, has also been suggested [10,11].
According to a meta-analysis of articles published between 2001 and 2007, the clinical presentation of thoracic endometriosis includes pneumothorax (72%), hemoptysis (14%), hemothorax (12%), and lung nodules (2%) [12]. Notably, most studies included in this meta-analysis involved a thoracoscopic examination. In our hospital, patients presented with hemoptysis in 53.3% of cases, and pneumothorax in 46.6%; a similar pattern was observed in the excluded patients.
Thoracic endometriosis is challenging in that diagnosis is extremely difficult, unless it is strongly suspected by experienced clinicians with a multi-modality approach. First, symptoms that precede or occur concurrently with menstrual bleeding are typical; however, symptoms that occur in the inter-menstrual period will not exclude the diagnosis of thoracic endometriosis. Thoracic endometriosis-related pneumothorax has even been reported during early pregnancy [13]. Second, patients commonly complain of chest pain, hemoptysis, dyspnea, cough, and scapular pain; these symptoms often accompany other more common pulmonary pathologies, particularly lung malignancies or tuberculosis, making differential diagnosis difficult. Two patients in this series had received empirical tuberculosis medication, before receiving a confirmative diagnosis. Third, there are no specific diagnostic modalities or diagnostic criteria, and in cases involving hemoptysis in particular, radiological abnormalities are transient. The CT findings for thoracic endometriosis may include ground glass opacities, nodular lesions, thin-walled cavities, or bullae [14].
The mechanism of right lung dominance in thoracic endometriosis has yet to be explained. A meta-analysis of 74 cases of catamenial hemoptysis, with diagnoses dating from 1956, revealed that 37 cases (59.6%) occurred in the right lung, and 19 cases (30.6%) occurred in the left lung. Six patients (9.7%) had bilateral lesions [11]. In our series, the vast majority of cased had right lung lesions only. A possible explanation for this phenomenon is that endometrial tissue may circulate clockwise, with the flow of peritoneal fluid in the abdominal cavity [15].
Histological confirmation of ectopic endometrium is not always performed, nor is it always possible. Previous definitions of thoracic endometriosis have specified that histological confirmation requires the presence of both endometrial stroma and glands; the presence of only stroma or pulmonary parenchymal hemorrhages and/or hemosiderin laden macrophages was considered suggestive [16]. These different patterns of histology were also evident in our series, and the overall clinical picture, in conjunction with the biopsy findings, should be considered for appropriate diagnosis.
The prevalence of concurrent pelvic endometriosis in patients with catamenial pneumothorax is reported to be anywhere between 18% and 84% [17,18]. According to our analysis, pelvic endometriosis was seen in 3/15 (20%) patients which is consistent with previous reports. A raised CA-125 was present only in those with co-existing pelvic endometriosis.
For women with thoracic endometriosis, surgery can provide relief of the associated chest symptoms. However, due to the high rate of recurrence and the invasiveness of most thoracic procedures, salvage surgery is strongly considered. The primary goal of surgery for thoracic endometriosis is to minimize recurrence by precisely locating the lesions and completely removing them, when all other conservative measures fail. In our study, the single patient that underwent bronchial artery embolization experienced no recurrence; this is consistent with a report from another institution [19], suggesting embolization may be suitable as a first line treatment. Because of the risks of thoracic surgery, operative management should be a last resort in cases where symptoms prevail. In these cases, minimally invasive, targeted resection, with routine adjuvant medical maintenance with a GnRH agonist, with or without progestins, should always be considered.
Postoperative recurrence rates are lower when adjuvant hormonal therapy is employed, the standard approach being a GnRH agonist for 3 to 6 months [20]. In our study, recurrence was not detected in any of the 6 cases that received adjuvant hormonal therapy after surgery, including the patient that underwent bronchial artery embolization, which has important therapeutic implications for management of thoracic endometriosis.
Due to the paucity of thoracic endometriosis itself, there is a lack of good controlled evidence for the use of adjuvant GnRH agonists or oral contraceptives. Also with dienogest (Visanne), there is no description on its effectiveness for thoracic endometriosis but taking its pharmacological mechanism into account, should be considered as a good option when patients are reluctant to continue with GnRH agonists or oral contraceptives. Further studies should be proceeded for the impact of thoracic endometriosis as with other extra-pelvic endometriosis.
In conclusion, the diagnosis and treatment of thoracic endometriosis requires a multidisciplinary approach and skillful differential diagnosis, based on a careful gynecological history and inquiry into the cyclicity of pulmonary symptoms. Although imaging findings are non-specific, laterality towards the right lung may be a feature. Since recurrence is more common in those presenting with a pneumothorax, extra caution is warranted after surgery, with a strong recommendation for adjuvant medical therapy (Fig. 2).
Figures and Tables
Fig. 1
Intraoperative photos from a patient presenting with catamenial hemoptysis (P4). (A) Multiple endometriotic spots and a hemorrhagic appearance, observed on the surface of the left upper lobe. (B) After lobectomy, the left upper lobe (dimensions, 18×12×2.5 cm; weight, 160 g) shows significant hemorrhagic areas. Intraoperative photos from a patient presenting with catamenial pneumothorax (P13). (C) Multiple 1 to 5 mm diaphragmatic holes are found on the central tendon of the diaphragm, with multiple endometriotic spots. (D) The specimen after diaphragmatic resection (dimensions, 5×3×0.3 cm). Histology of the resected diaphragm showed the multiple nodules and endometrial stromal cells.
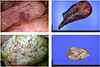
Fig. 2
Algorithm summarizing thoracic endometriosis management protocols employed at our institute. BAE, bronchial artery embolization.

Table 3
Treatments and prognosis in the patients with thoracic endometriosis

CH, thoracic endometriosis with catamenial hemoptysis; VATS, video-assisted thoracoscopic surgery; WR, wedge resection; LUL, left upper lobe; BAE, bronchial artery embolization; DR, diaphragmatic resection; MPA, medroxyprogesterone acetate; OC, oral contraceptive.
a)Number of episode after initial treatment; b)Drospirenone 3 mg ethynyl estradiol 0.03 mg.
References
1. Attaran M, Falcone T, Goldberg J. Endometriosis: still tough to diagnose and treat. Cleve Clin J Med. 2002; 69:647–653.
2. Szamatowicz M. Endometriosis: still an enigmatic disease. What are the causes, how to diagnose it and how to treat successfully? Gynecol Endocrinol. 2008; 24:535–536.
3. Augoulea A, Lambrinoudaki I, Christodoulakos G. Thoracic endometriosis syndrome. Respiration. 2008; 75:113–119.
4. Hilaris GE, Payne CK, Osias J, Cannon W, Nezhat CR. Synchronous rectovaginal, urinary bladder, and pulmonary endometriosis. JSLS. 2005; 9:78–82.
5. Visouli AN, Darwiche K, Mpakas A, Zarogoulidis P, Papagiannis A, Tsakiridis K, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis. 2012; 4:Suppl 1. 17–31.
6. Simoglou C, Zarogoulidis P, Machairiotis N, Porpodis K, Simoglou L, Mitrakas A, et al. Abdominal wall endometrioma mimicking an incarcerated hernia: a case report. Int J Gen Med. 2012; 5:569–571.
7. Veeraswamy A, Lewis M, Mann A, Kotikela S, Hajhosseini B, Nezhat C. Extragenital endometriosis. Clin Obstet Gynecol. 2010; 53:449–466.
8. Bagan P, Berna P, Assouad J, Hupertan V, Le Pimpec Barthes F, Riquet M. Value of cancer antigen 125 for diagnosis of pleural endometriosis in females with recurrent pneumothorax. Eur Respir J. 2008; 31:140–142.
9. Haga T, Kataoka H, Ebana H, Otsuji M, Seyama K, Tatsumi K, et al. Thoracic endometriosis-related pneumothorax distinguished from primary spontaneous pneumothorax in females. Lung. 2014; 192:583–587.
10. Okeke TC, Ikeako LC, Ezenyeaku CC. Endometriosis. Niger J Med. 2011; 20:191–199.
11. Huang H, Li C, Zarogoulidis P, Darwiche K, Machairiotis N, Yang L, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res. 2013; 18:13.
12. Channabasavaiah AD, Joseph JV. Thoracic endometriosis: revisiting the association between clinical presentation and thoracic pathology based on thoracoscopic findings in 110 patients. Medicine. 2010; 89:183–188.
13. Yoshioka H, Fukui T, Mori S, Usami N, Nagasaka T, Yokoi K. Catamenial pneumothorax in a pregnant patient. Jpn J Thorac Cardiovasc Surg. 2005; 53:280–282.
14. Orriols R, Munoz X, Alvarez A, Sampol G. Chest CT scanning: utility in lung endometriosis. Respir Med. 1998; 92:876–877.
15. Suginami H. A reappraisal of the coelomic metaplasia theory by reviewing endometriosis occurring in unusual sites and instances. Am J Obstet Gynecol. 1991; 165:214–218.
16. Rousset-Jablonski C, Alifano M, Plu-Bureau G, Camilleri-Broet S, Rousset P, Regnard JF, et al. Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum Reprod. 2011; 26:2322–2329.
17. Tripp HF, Obney JA. Consideration of anatomic defects in the etiology of catamenial pneumothorax. J Thorac Cardiovasc Surg. 1999; 117:632–633.
18. Joseph J, Sahn SA. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996; 100:164–170.
19. Shin SP, Park CY, Song JH, Kim HM, Min D, Lee SH, et al. A case of catamenial hemoptysis treated by bronchial artery embolization. Tuberc Respir Dis. 2014; 76:233–236.
20. Lee DY, Bae DS, Yoon BK, Choi D. Post-operative cyclic oral contraceptive use after gonadotrophin-releasing hormone agonist treatment effectively prevents endometrioma recurrence. Hum Reprod. 2010; 25:3050–3054.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download