Abstract
Epithelial borderline ovarian tumors (BOT) are distinctive from benign tumors and carcinoma. They occur in younger women more often than carcinoma, and there is some difficulty making correct diagnosis of BOT. Two subtypes of BOT, serous and mucinous borderline tumor have different characteristics and very different clinical behavior. Serous borderline tumor (SBT) with micropapillary pattern shows more incidence of extra ovarian disease and often coexists with invasive implant. SBT with micropapillary pattern in advanced stage has showed a worse prognosis than typical SBT. Huge mucinous borderline tumors have histologic heterogeneity, and the accuracy of frozen section diagnosis is relatively low. Extensive sampling is required to reach a correct pathological diagnosis. Mucinous adenoma (intestinal type) also runs the risk of recurrence after cystectomy, or intraoperative rupture of cyst. Laparoscopic procedure for BOT has not increased the risk of recurrence. Fertility preserving procedures are generally accepted, except in advanced stage SBT with invasive implants. Only cystectomy shows a significant risk of recurrence. Re-staging surgery and full staging surgery is not necessary for all BOT. We should not attempt to treat them uniformly, by the single diagnosis of "borderline tumor". It depends on histologic type. Close communication with the pathologist is necessary to gain more detail and ask more pathological samples in order to make the optimal treatment strategy for each individual patients.
Borderline ovarian tumor (BOT) was first described in 1929 by Taylor [1], as the tumor having a pathological and clinical intermediate form, between benign and malignant ovarian tumor. The histology of BOT was defined by the World Health Organization as that exhibiting atypical epithelial proliferation, greater than seen in benign counterparts without destructive stromal invasion. Classifications for these tumors have since been modified. Compared to carcinoma, they occur more frequently in younger women, so different treatment strategies should be considered.
The incidence of BOT in Japan is 23.6% of non-benign ovarian tumor (Japan Society of Obstetrics and Gynecology tumor committee 2012). The most two common histologic subtypes are serous and mucinous BOT. The histologic distribution of BOT shows geographic variation. In western countries, such as the USA, Germany, and Italy, the serous is the most frequent, at over 70% of all BOT, but in most Asian countries, the mucinous is more dominant, and in some other European countries, such as Scandinavian and Spain, both tumors occur with similar frequency [2].
Serous borderline tumor (SBT) is diagnosed by the stratified serous epithelial cells resembling the fallopian tube with a hierarchical pattern of branching and a varying degree of nuclear atypia with an absence of frank invasion. Some portion of the tumor detaches into a cyst. Bilateral tumor represents about 30% of all cases, and in 70% of cases, the tumor is limited to the ovary.
There are some variations of SBT with possibly worse prognostic features including micropapillary pattern (MP), microinvasion, extraovarian implant, lymphnode involvement, and bilateral tumor. These findings are related to worse prognoses, and these parameters often coexist within an individual tumor. SBT-MP shows a non-hierarchial pattern with micropapillary projection 5 times longer than wide, at least 5 mm in dimension, with prominent fibrous stalks and cribriform pattern. In SMT-MP, surface involvement, bilateral, and peritoneal implants are found more frequently than in usual serous BOT. Several studies have revealed that SBT-MP shows a worse prognosis than usual SBT, especially in stage II or more advanced stage [3].
Extraovarian disease is referred to as implant, and implants are found in 20% to 40% of SBT, mostly in the omentum or peritoneal surface. Most cases (88%) are non-invasive implants, which are subdivided into desmoplastic implant and epithelial implant. Defined by the limits of surrounding tissue and whether or not the adhesion can be slipped off easily (Fig. 1). Invasive implants occur less frequently (12%), but exhibit destructive invasion of underlying tissue. The tumor resembles low-grade serous adenocarcinoma and shows a significantly worse prognosis [3]. Lymph node involvement was found to occur in 20% to 30% of cases when lymph node dissection was performed. Most cases are microscopic, without nodal enlargement, and mostly seen with extraovarian inplants. Endosalpingiosis occur in lymph node, and should not be confused with lymph node involvement. Different from invasive cancer, the clinical significance of lymph node involvement in SBT is not clear. There is no definite survival difference between diagnosis of node positive and negative, suggesting lymph node involvement is via the peritoneal route, not through lymphatic channels [4].
Although, the vast majority of serous carcinoma arise de novo, 5% to 10% of serous carcinoma derived from the precursor lesion SBT [5]. The prognosis of SBT is excellent (98% in 5-year survival) in stage I tumor patients who received comprehensive staging surgery, and patients with advanced stage non-invasive implant also have a 90% of 5-year survival rate. Patients with invasive implants, however, have only 66% 5-year survival rate. There is no proven benefit of adjuvant chemotherapy for advanced stage patients. Long term follow up is necessary, and there is a small risk of transformation to high grade serous carcinoma. Fertility preserving surgery should be considered for younger patients, although it should be noted that a high recurrence risk dose exist in case with invasive implant [6].
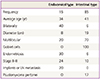
Mucinous borderline tumor (MBT) is diagnosed by the proliferation of mucinous epithelial tumor cells with intermediate (variable) nuclear atypia and an absence of frank stromal invasion. The incidence of MBT is 10% of all mucinous tumors and 40% to 50% of all non-benign mucinous tumors. There are two major subtypes, classified as the intestinal type (85%) and the endocervical type (15%), and they have different clinical features. Intestinal MBT (IMBT) occurs in relatively older women (40 to 50 years old). They are usually unilateral, large (19 cm in diameter) multilocular cysts, and in most cases are limited to the ovary. Microscopically, they have mucin containing gastrointestinal type cells with goblet cells or Paneth cells, stratified into two or three layers. Often these tumors contain areas of cystadenoma and noninvasive carcinoma. Endocervical like type MBT (EMBT) occurs in younger women (35 to 39 years old), are relatively small (8 to 10 cm), and 40% are bilateral, macroscopically unilocular cystic tumors. Microscopically, they have mucin containing cells resembling endocervical cells showing mild to focally severe atypia. Stage II to III tumors are more frequently found in EMBT than IMBT (Table 1) [5]. These two mucinous tumors should be discriminated absolutely because of their different clinical features [7]. Seromucinous BOT are composed of a mixture of EMBT (most common), SBT, endometrioid tumor, or squamous cell tumor. Early on, these tumors were called as Müllerian mucinous borderline tumors, Müllerian mixed borderline tumors, or mixed epithelial borderline tumor. They became an independent entity in new World Health Organization criteria. They now have clinical features of serous and mucinous tumor, but the serous element decides the clinical behavior such as SBT-MP or invasive implant [8].
There are some adverse prognostic histological variations in MBT. Intraepithelial carcinoma exhibit severe nuclear atypia in the absence of invasion. Microinvasion is defined as limited invasion, within 3 to 5 mm, and less than 10 mm2 of foci.
In MBT, the result of frozen section often does not yield correct histology. Discordant diagnosis occurs in 34% of MBT. A factor which has the potential to influence accuracy of diagnosis by frozen section is tumor size of more than 10 cm, and borderline element contents of less than 10%. If the tumor weight is over 1,360 g, the discordant rate elevates up to 50%. Most (94%) cases of discordant diagnosis occur in tumors with a size of more than 13 cm [9,10].
Prognosis of MBT is excellent in stage I tumor. Nevertheless, 9% of MBT, especially cases of IMBT, recurred within 5 years and 13% recurred within 10 years, in the form of mucinous carcinoma. Even benign mucinous adenoma rarely recurred after cystectomy, or intraoperative rupture of cyst. Cystectomy is the only adverse prognostic factor. More than 80% of invasive mucinous carcinoma contained areas of IMBT and intraepithelial carcinoma as precursor lesions [7]. High heterogeneity (benign, borderline, intraepithelial carcinoma, microinvasion, and invasive carcinoma) are presented in huge mucinous tumor, especially in intestinal type. Most recurrent cases have the possibility of sampling error at the tumor resection [11]. So, adequate pathologic sampling is mandatory to detect a small focus of carcinoma, in cases of borderline tumor is suspected. One section per cm in tumors under 10 cm, and two sections per cm for tumors over 10 cm should be obtained (National Cancer Institute ovarian tumor workshop 2003) [12]. In addition, more sections are needed, if the tumor has aggressive proliferation or nuclear atypia. Recurrence of mucinous adenoma within a short period should be treated by adnexectomy and a re-check of the primary surgical specimen [13]. We had one case of mucinous cyst adenoma intestinal type who recurred only 6 month after cystectomy. Full sectioning of the recurrent tumor revealed MBT intestinal type, and also a small portion of carcinoma was detected after re-reviewed the specimen of the primary tumor (Fig. 2).
Laparoscopy has become the standard technic for benign ovarian tumor. Because of the low accuracy of preoperative correct diagnoses of MBT, laparoscopic surgery is often employed. Laparoscopy has the potential risk of recurrence such as rupture of cyst, tumor cell dissemination, and trocar site metastasis. Nevertheless, there is no association with a worse prognosis between laparotomy and laparoscopy. Most cases of recurrence with laparoscopy are conservatively treated [14]. If unexpected borderline histology is found after primary surgery, re-staging surgery may be planned. The incidence of up-staged cases with re-staging surgery make up 10% to 30% of SBT by MP pattern or invasive implant, and 4% to 15% of MBT. The site of disease is found more in the omentum or pelvic peritoneum, and less often in the opposite ovary or pelvic node. It is still controversial, whether or not re-staging surgery contributes to prognosis. There was no significant difference in patient survival between patients who underwent re-staging surgery and those who did not. Procedures of re-staging surgery for women wishing fertility preserving include careful inspection of peritoneum, random biopsy at peritoneum, infracolonic omentectomy, appendectomy (MBT), washing cytology, and lymphadenectomy for cases with suspicious invasive. Unlike invasive cancer, systemic lymphadenectomy is not always necessary and it depends on histologic type. In cases of SBT-MP, lymphadenectomy should be required, but in cases of intestinal MBT, it may not be [15]. Fertility preserving surgery is an appropriate strategy in young women with BOT, except in cases with advanced stage with invasive implant. Cystectomy has the highest risk of recurrence, and long term follow up is necessary.
In conclusion, BOT consists of two different types, SBT and MBT. Each type shows different characteristics, and each has unique subclassifications and clinical features which influence prognosis. Accordingly, we should not attempt to treat them uniformly, by the single diagnosis of "borderline tumor." To avoid inappropriate (inadequate or over) treatment, close communication with the pathologist is necessary to gain more detail and ask more pathological samples in order to make the optimal treatment strategy for each individual patients.
Figures and Tables
Fig. 1
(A) Stage IIIb serous borderline tumor with non-invasive implant. (B) Multiple adhesions in pelvic cavity and papillary tumor existed around the ovary and the peritoneum, but adhesiolysis is done easily.

Fig. 2
Histology of a case of mucinous adenoma recurrence after cystectomy. (A) Primary tumor: mucinous adenoma intestinal type (×100). (B) At recurrence, mucinous borderline tumor intestinal type was detected after full sectioning (×200). (C) A small portion of intraepithelial carcinoma was found after re-reviewed primary tumor (×100).

References
1. Taylor HC. Malignant and semimalignant tumors of the ovary. Surg Gynecol Obstet. 1929; 48:204–230.
2. Song T, Lee YY, Choi CH, Kim TJ, Lee JW, Bae DS, et al. Histologic distribution of borderline ovarian tumors worldwide: a systematic review. J Gynecol Oncol. 2013; 24:44–51.
3. Bell DA, Longacre TA, Prat J, Kohn EC, Soslow RA, Ellenson LH, et al. Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum Pathol. 2004; 35:934–948.
4. Fadare O, Orejudos MP, Jain R, Mariappan MR, Hecht JL, Renshaw IL, et al. A comparative analysis of lymphatic vessel density in ovarian serous tumors of low malignant potential (borderline tumors) with and without lymph node involvement. Int J Gynecol Pathol. 2008; 27:483–490.
5. Prat J. Pathology of the ovary. Philadelphia: Saunders;2004.
6. Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000; 31:539–557.
7. Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005; 24:4–25.
8. Shappell HW, Riopel MA, Smith Sehdev AE, Ronnett BM, Kurman RJ. Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas. Am J Surg Pathol. 2002; 26:1529–1541.
9. Brun JL, Cortez A, Rouzier R, Callard P, Bazot M, Uzan S, et al. Factors influencing the use and accuracy of frozen section diagnosis of epithelial ovarian tumors. Am J Obstet Gynecol. 2008; 199:244.e1–244.e7.
10. Storms AA, Sukumvanich P, Monaco SE, Beriwal S, Krivak TC, Olawaiye AB, et al. Mucinous tumors of the ovary: diagnostic challenges at frozen section and clinical implications. Gynecol Oncol. 2012; 125:75–79.
11. Koskas M, Uzan C, Gouy S, Pautier P, Lhomme C, Haie-Meder C, et al. Prognostic factors of a large retrospective series of mucinous borderline tumors of the ovary (excluding peritoneal pseudomyxoma). Ann Surg Oncol. 2011; 18:40–48.
12. Chiesa AG, Deavers MT, Veras E, Silva EG, Gershenson D, Malpica A. Ovarian intestinal type mucinous borderline tumors: are we ready for a nomenclature change? Int J Gynecol Pathol. 2010; 29:108–112.
13. Ben-Ami I, Smorgick N, Tovbin J, Fuchs N, Halperin R, Pansky M. Does intraoperative spillage of benign ovarian mucinous cystadenoma increase its recurrence rate? Am J Obstet Gynecol. 2010; 202:142.e1–142.e5.
14. Morice P, Uzan C, Fauvet R, Gouy S, Duvillard P, Darai E. Borderline ovarian tumour: pathological diagnostic dilemma and risk factors for invasive or lethal recurrence. Lancet Oncol. 2012; 13:e103–e115.
15. Fauvet R, Boccara J, Dufournet C, David-Montefiore E, Poncelet C, Darai E. Restaging surgery for women with borderline ovarian tumors: results of a French multicenter study. Cancer. 2004; 100:1145–1151.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download