Abstract
Objective
Human papillomavirus (HPV) test was incorporated into the triage of lesser abnormal cervical cytologies: atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL). This study aimed to evaluate the impact of age on the efficacy of HPV testing in patients with lesser abnormal cervical cytologies.
Methods
A total of 439 patients with ASCUS or LSIL were included. The association between age groups and the diagnostic performances of HPV test for high-grade cervical intraepithelial neoplasia (CIN2+) was evaluated.
Results
Median age was 44 years (range, 17 to 75 years). ASCUS was more frequently observed in older patients while LSIL was more common in younger patients (P=0.002). CIN2+ was found in 11.3% (32/284) of the ASCUS patients and 12.9% (20/155) of patients with LSIL. Older patients with ASCUS showed lower HPV infection rates (P=0.025), but not LSIL (P=0.114). However, the prevalence of CIN2+ was similar between the age groups with ASCUS or LSIL. In patients with ASCUS, the false negative rate of HPV test for CIN2+ was 6.2%. The false negative rate of the HPV test became higher with increasing of the age after the age of 50 (P=0.034).
Women with lesser abnormal cervical cytological lesions identified in a Pap smear such as atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) have a small but significantly increased risk of developing cervical cancer compared to women with a normal finding on Pap. However, in contrast to the reproducible cytologic classification of LSIL, ASCUS is often an equivocal result and, for that reason, the most common cytologic abnormality, however, the least reproducible [1,2]. To improve the management of women with ASCUS cytology, many studies tried to demonstrate the cost-effectiveness of human papillomavirus (HPV) testing for triage of ASCUS cytology [3,4]. The proven cost-effectiveness of HPV testing in ASCUS triage led to the 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors [5]. According to the guidelines, colposcopy is indicated for all women with HPV-positive ASCUS regardless of the genotyping results, because the 5-year cumulative risk for cervical intraepithelial neoplasia (CIN) 2 or worse (CIN2+) among women testing HPV-positive ASCUS was similar to that of women with LSIL (18% vs. 16%) [6].
By contrast, HPV-negative ASCUS is recommended to be followed with co-testing at 3 years, because the risks of CIN2+ were similar between HPV-negative ASCUS and negative Pap alone. However, the similarity was found only for women under the age of 60 years, after which cancer risk increased for HPV-negative ASCUS versus negative Pap-alone results (aged 60 to 64 years, 0.26% vs. 0.035%, respectively; P=0.3) [6]. A recent epidemiologic study reported that age was a strong determinant of high-risk HPV infection in Korean women with a significant lower infection rate in older than in younger women [7]. There was considerable evidence for the suggested significant impact of age on HPV triage of ASCUS cytology for colposcopy referral [8]. As such, age could be another criterion for improving HPV triage of ASCUS. Nonetheless, there was no study that evaluates the age impact on the diagnostic performance of HPV test in lesser abnormal cytologies in Korean women. We, therefore, investigated the impact of age on the diagnostic performance of the HPV test in patients with ASCUS/LSIL in Korean women.
In our retrospective study, a total of 439 consecutive patients who had ASCUS or LSIL results on Pap smear and underwent colposcopic biopsy for the cytologic abnormalities at the Seoul National University Bundang Hospital between January 2010 and December 2011 were included. Women without corresponding biopsy results were excluded. All the patients had the corresponding HPV DNA testing results, either by hybrid capture or by HPV genotyping, within 2 months before cervical biopsy. Those who had undergone hysterectomy and had a past medical history of cervical cancer or CIN were excluded. Women who were pregnant at the time of cervical cytology were also excluded. The study protocol was approved by the ethics committee of the Seoul National University Bundang Hospital. Clinicopathologic variables of the patients including age at the time of colposcopy, the results of abnormal cervical cytologies, HPV DNA test, and punch biopsy were obtained from review of the electronic medical record system. The patients were grouped into five according to the age: ≤30, 31 to 40, 41 to 50, 51 to 60, and >60 years.
Cytological diagnoses were classified using the 2001 Bethesda System Nomenclature. Hybrid capture II HPV test (HC II; Digene Corporation, Gaithersburg, MD, USA) and GG HPV DNA genotyping chip kit (Goodgene, Seoul, Korea) were used for detecting high-risk HPV infection according to the physician's preference. HC II testing was performed no later than 5 days after the collection of the specimens according to the manufacturer's instructions [9]. The specimens with relative light units/cutoff value ratios >1.0 were considered positive for one or more high-risk HPV genotypes including the following 13 genotypes: HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. Another method we used for detecting high-risk HPV infection was the GG HPV DNA genotyping chip kit (Goodgene). This method identifies 40 HPV types including 15 high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 69). According to the manufacturer's instructions, genomic DNA extraction, amplification, labeling and hybridization as well as analysis were performed.
Colposcopy with biopsy was performed within 2 weeks after having obtained the above named cytology results. Ectocervical biopsies of visible lesions after application of 3% acetic acid or 4 locations (3, 6, 9, and 12 o'clock direction), if no lesion was visible, were obtained. Endocervical curettage was also performed in women with no lesions. Biopsy specimens were reviewed by an experienced gynecologic pathologist and diagnosed using standard criteria and the CIN terminology.
Chi-square test or Fisher's exact test, when appropriate, were used for comparing proportions and identifying any statistical significance of the difference in the sensitivity and specificity of HPV test between age groups. Test for trend by linear by linear association was used for the analysis according to the five age groups. Two-sided P<0.05 was considered statistically significant. The SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis.
Patient characteristics are shown in Table 1. Median age was 44 years (17 to 75 years). ASCUS was found in 284 (64.7%) and LSIL was found in 155 (35.3%). High-risk HPV was positive in 276 (62.9%) patients. CIN 1, 2, and 3 were diagnosed in 106 (24.1%), 29 (6.6%), and 18 (4.1%), respectively. Highrisk HPV infection rate was increased as the severity of CIN lesions on punch biopsy results: 54.1% of patients with negative punch biopsy result, 69.8% of CIN1, 93.1% of CIN2, and 100% of CIN3+. Five (1.1%) had a diagnosis of invasive carcinoma on punch biopsy.
ASCUS was more frequently observed in older patients, while LSIL was more common in younger patients (P=0.002) (Table 2). High-risk HPV infection was more frequently observed in younger patients than older patients (P=0.027). Nevertheless, the proportion of CIN2+ was similar between the age groups (P=0.614). In two separate subgroup analyses of patients with ASCUS (Table 3) and LSIL (Table 4), the decreasing trend of high-risk HPV infection in older women was observed in the patients with ASCUS (P=0.025), but not in the patients with LSIL (P=0.114). The prevalence of CIN2+ lesions was not different between the age groups not only in the patients with ASCUS (P=0.303) but also in the patients with LSIL (P=0.860).
In patients with ASCUS, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the HPV test for CIN2+ lesions were 93.8%, 38.5%, 16.2%, 98.0%, and 37.7%, respectively (Table 5). In patients with LSIL, the corresponding values of HPV test for CIN2+ lesions were 100%, 47.4%, 22.0%, 100%, and 54.2%, respectively. After grouping the patients with ASCUS into five age groups, the first three age groups (≤30, 31 to 40, 41 to 50 years) had a false negative rate 0%. After the age of 50 years, the increasing trend of the false negative rate in older women was observed (χ2=4.511, P=0.034). However, the false negative rates of the two oldest age groups were not significantly higher than that of the youngest age group (16.7%, 33.3%, and 100% for 51 to 60, >60, and ≤30 years, respectively, P=0.999). By contrast, the sensitivity of 100% of HPV test for CIN2+ lesions in women with LSIL was not changed in every five age groups. The specificities of older age groups were not higher than that of age ≤30 years in women with ASCUS and LSIL (Table 5). Any increasing trend of specificity was not observed in older women with ASCUS (χ2=2.975, P=0.085) and LSIL (χ2=2.997, P=0.083).
In this study we evaluated the impact of age on the HPV triage in patients with lesser abnormal cervical cytologies. The more severe the CIN lesion was, the more frequently a high-risk HPV infection was observed. Despite the lower rate of high-risk HPV infection in older than in younger patients, there was no difference of CIN2+ lesions between the age groups, this trend was observed in ASCUS, but not LSIL. In contrast to the consistent sensitivity of 100% of high-risk HPV test for CIN2+ lesions in every age group with LSIL, the sensitivity of 100% in young patients ≤30 years with ASCUS decreased to 66.7% in old patients aged >60 years. In other words, the false negative rate of older patients >60 years with ASCUS was as high as 33.3%, compared to 0% in the youngest counterpart.
The problem of the HPV test as the preferred option for ASCUS triage is the poor specificity for CIN2+ lesions, which could lead to over-diagnosis of regressive CIN2+, subsequently excessive colposcopy referral, particularly in younger women [10]. There are consistent data of higher prevalence of highrisk HPV infection in younger women than the older counterpart. Many HPV infections in women under age 30 are recently acquired infections, most of which will naturally clear in a few years without progressing, even in case of CIN 2, thus HPV triage may be less efficient for those women [11]. Poor specificity of a positive HPV test for CIN2+ lesions in younger women in our study was also confirmed in a recently published population-based study from Norway, in which the specificity was 47%, 71%, and 82% for the age groups <34, 34 to 50, and >50 years, respectively [12].
As the age advanced, however, the specificity increased whereas the sensitivity decreased. These findings were consistent with those of the study by Stoler et al. [13] reporting that the sensitivity of HPV test declined dramatically with increasing age, 93.3% in 21 to 29 years and 67.7% in 40 years or older, whereas specificity improved. They explained that the decreased sensitivity was most likely owing to pathologic misclassification of non-CIN lesions as CIN2+ lesions in older women because of age-related mimics. Furthermore, they mentioned that increased numbers of a false negative HPV test results might occur like in our study [13]. The false negative rate of the HPV test in ASCUS/LSIL has been reported as 3.7% to 18.2% [14,15,16]. Jastania et al [17]. reported the characteristics of false negative HC II testing. They stated that HPV DNA was present in the assessed samples, but the level of viral DNA was less than the amount necessary for the result to be considered positive. They also mentioned unsatisfactory cytologic sample for evaluation virtually devoid of cells as a possible reason because there were no criteria for evaluating the adequacy of specimens for HC II testing when using specimens obtained separately from a liquid-based cytologic sample just like in our study. By contrast, there were a few studies demonstrating that the sensitivity for detection of CIN2+ was not affected by age [8,12,18].
According to the recently published results of a large-scale demonstration project of HPV triage of ASCUS using data from a retrospective cohort of the Kaiser Permanente Northern California (KPNC) database, HPV-negative ASCUS had substantially higher CIN3+ risks than an HPV-negative/Pap-negative result, indicating that an ASCUS Pap may convey some risk information in the absence of detectable HPV [6]. Although they noted a strong decline in HPV positivity in ASCUS by increasing age of women being tested as observed in our study, this study failed to show the expected age-dependent decreases of CIN2+ risks for patients with HPV-negative ASCUS or HPV-positive ASCUS. These findings may support the explanation of age-related pathologic misclassification by Stoler et al. [13] Instead, cancer risk seemed to be relatively steadily rising in women with HPV-positive ASCUS after the age 35 to 39 including a striking increase in age 60 to 64. The abrupt increase of the cancer risk was also found in women with HPV-negative ASCUS aged 60 to 64. The increased cancer risk and the increased false negative rate of HPV test for detecting CIN2+ lesions in older women with ASCUS cytology suggest that HPVnegative ASCUS findings should be further investigated and not used instead of negative Pap results to qualify old women, especially for women aged over 60, to exit screening [6]. The 2012 American Society for Colposcopy and Cervical Pathology guidelines in the USA recommended that HPV-negative ASCUS in women aged ≥65 requires additional surveillance with repeat co-testing in 1 year [5]. Our study supports this special consideration for the old age group based on our findings of aberrantly poor diagnostic performances of HPV test for CIN2+ lesions, including sensitivity, specificity, positive predictive value, and negative predictive value, in women aged over 60 with ASCUS cytology.
In the previous studies, the rate of CIN2+ has been reported as follows: 3.6% to 6.9% in ASCUS and 8.7% to 16% in LSIL [6]. The rate of CIN2+ (11.3%) in ASCUS patients in the present study appears somewhat higher than those of previous studies. Ethnic difference of the incidence of cervical premalignant lesions, different sensitivities of HPV tests that used in the studies, and a certain degree of selection bias in a retrospective study could be considered as plausible reasons for this differdifference of CIN2+ prevalence in ASCUS women.
In relation to the possible existence of a selection bias, we did not exclude unsatisfactory sampling of our false negative cases. However, in contrast to 0% in the younger patients group, the false negative rate of 12.5% in the elder group might not be fully explained just by methodological errors. It might be another limitation of this study that small sample size, particularly 0 cells in the analysis after age grouping, caused difficulties in calculating accurate P-values as well as lowered the statistical power. Lastly, two different HPV DNA tests were used for detecting high-risk HPV infection. Nevertheless, the two methods were reported to have similar diagnostic performances.
In conclusion, our findings suggest that false negative rate of the HPV test for CIN2+ in ASCUS patients older than 50 years might become higher with increasing of the age. Considering the potential increase of cancer risk in old women as well as the ethnic preponderance of cervical cancer in Korean women, negative results of HPV tests in the old patients with ASCUS cytology should be carefully interpreted.
Figures and Tables
Table 3
Comparison of the prevalence of high-risk HPV and CIN2+ lesion between age groups in patients with ASCUS (n=284)

Table 4
Comparison of the prevalence of high-risk HPV and CIN2+ lesion between age groups in patients with LSIL (n=155)

Table 5
Age impact on the diagnostic performance of HPV DNA test for CIN2+ lesions
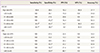
The scale of age is year.
HPV, human papillomavirus; CIN, cervical intraepithelial neoplasia; PPV, positive predictive value; NPV, negative predictive value; ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion.
P-values by test for trend by linear by linear association: a)0.098 (Fisher's exact test); b)0.034 and by chi-square test compared with age ≤30, 0.999 (Fisher's exact test); c)0.116; d)0.340; e)0.128; f)0.258; g)0.538 (Fisher's exact test).
References
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998; 92:727–735.
2. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risks of CIN 2+ and CIN 3+ among women with HPV-positive and HPV-negative LSIL Pap results. J Low Genit Tract Dis. 2013; 17:S43–S49.
3. Kulasingam SL, Kim JJ, Lawrence WF, Mandelblatt JS, Myers ER, Schiffman M, et al. Cost-effectiveness analysis based on the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion Triage Study (ALTS). J Natl Cancer Inst. 2006; 98:92–100.
4. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002; 287:2382–2390.
5. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 Updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013; 17:S1–S27.
6. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV testing of ASC-US Pap results. J Low Genit Tract Dis. 2013; 17:S36–S42.
7. Kim K, Kim JJ, Kim SM, No JH, Kim YB. Prevalence and determinants of high-risk human papillomavirus infection in women with high socioeconomic status in Seoul, Republic of Korea. Asian Pac J Cancer Prev. 2012; 13:269–273.
8. Sherman ME, Schiffman M, Cox JT. Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study Group. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst. 2002; 94:102–107.
9. Poljak M, Brencic A, Seme K, Vince A, Marin IJ. Comparative evaluation of first- and second-generation digene hybrid capture assays for detection of human papillomaviruses associated with high or intermediate risk for cervical cancer. J Clin Microbiol. 1999; 37:796–797.
10. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010; 11:249–257.
11. Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstet Gynecol. 2007; 109:1325–1331.
12. Budal EB, Haugland HK, Skar R, Maehle BO, Bjorge T, Vintermyr OK. HPV DNA testing improves CIN2+ risk stratification and detection of CIN2+ in delayed triage of ASCUS and LSIL: a population-based follow-up study from Western Norway. Cancer Med. 2014; 3:182–189.
13. Stoler MH, Wright TC Jr, Sharma A, Zhang G, Apple R, Wright TL, et al. The interplay of age stratification and HPV testing on the predictive value of ASC-US cytology. Results from the ATHENA HPV study. Am J Clin Pathol. 2012; 137:295–303.
14. Solomon D, Schiffman M, Tarone R;. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001; 93:293–299.
15. Guo M, Hu L, Baliga M, He Z, Hughson MD. The predictive value of p16 (INK4a) and hybrid capture 2 human papillomavirus testing for high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2004; 122:894–901.
16. De Cremoux P, Coste J, Sastre-Garau X, Thioux M, Bouillac C, Labbe S, et al. Efficiency of the hybrid capture 2 HPV DNA test in cervical cancer screening: a study by the French Society of Clinical Cytology. Am J Clin Pathol. 2003; 120:492–499.
17. Jastania R, Geddie WR, Chapman W, Boerner S. Characteristics of apparently false-negative digene hybrid capture 2 high-risk HPV DNA testing. Am J Clin Pathol. 2006; 125:223–228.
18. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med. 2003; 127:946–949.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download