Abstract
A first-trimester ultrasound scan has become an essential part of antenatal care. The Korean Society of Ultrasound in Obstetrics and Gynecology held a first-trimester ultrasound forum on April 5, 2014. The forum aimed to present an updated review of the literature on the topic of first-trimester ultrasound in specific lectures and to host a panel discussion on several important issues regarding first-trimester scans. The forum provided evidence- and consensus-based best practice patterns for obstetricians in Korea. Here, we report the review and checklists presented from the forum.
A first-trimester ultrasound scan has become an essential part of antenatal care. The Korean Society of Ultrasound in Obstetrics and Gynecology (KSUOG) held a first-trimester ultrasound forum on April 5, 2014. The specific goal of this forum was to present an updated review of the literature on the topic of first-trimester ultrasound in specific lectures and to host a panel discussion with active communication among experts on several important issues regarding first-trimester scans. Also, optimal checklists and the plane for the measurement of nuchal translucency (NT) were reminded to the audience during this forum as shown in Table 1 and Fig. 1, respectively. Finally, this forum provided evidence- and consensus-based best practice patterns for obstetricians in Korea. We hope this review and checklists from the forum is helpful to every obstetrician's practice in Korea.
Using ultrasound to measure fetal NT in the first trimester is an effective method to screen for major chromosomal abnormalities [1,2,3,4]. When ultrasound is combined with maternal serum pregnancy-associated plasma protein A (PAPP-A), and free β-human chorionic gonadotropin, the sensitivity of first-trimester NT screening exceeds 80% at a screen positive rate of 5% [3,5]. When the measured NT is enlarged, karyotyping and detailed fetal ultrasonography, including echocardiography, should be performed. Recently published studies on outcome for fetuses with increased NT used different cutoff values: 3.0 mm, 95th percentile, and 99th percentile [1,2,6,7,8]. Therefore, optimal cutoff values can be considered by individual practitioners. The relationships between different cutoff values and the prevalence of chromosomal defects, fetal death, and major fetal abnormalities should be determined [9]. Our recent survey including KSUOG members found that 52.6% (101/192) use 3.0 mm of NT, 26.0% (50/192) use the 95th percentile of NT, and 21.4% (41/192) use 2.5 mm as an optimal cutoff value for diagnosis of increased NT. This indicates the need for diverse research to determine an optimal cutoff value of NT for the Korean population.
Combining fetal NT with maternal serum screening in the first trimester is more effective than using the NT measurement alone. Because there is not yet a general consensus on a valid cutoff value for NT, values such as 3.0 mm, 95th percentile, and 99th percentile are considered by individual practitioners as per the medical standard.
Over the years, many studies have shown that increased NT is associated with chromosomal abnormalities, congenital heart defects, genetic syndromes, a higher risk of miscarriage, and intrauterine fetal death [5,6,7,8,9,10]. Moreover, chromosomally normal fetuses with an increased NT are reported to have a higher incidence of structural anomalies, mainly cardiac, in addition to increased risk for an adverse outcome [6,7,8,9,10]. The chance of an uneventful pregnancy outcome is inversely related to the initial degree of enlargement. Many studies have reported that, when fetuses with increased NT have normal karyotype and normal fetal anatomy (as proven by detailed fetal ultrasonography), pediatric outcomes are as good as would be expected in the general population [11,12,13,14,15]. Without a firm consensus on how to counsel parents of a euploid fetus with enlarged NT, talking with parents in this situation is difficult.
Nonetheless, patients and their families can be told that, in pregnancies where the increased NT resolves and detailed ultrasound examinations reveal no additional anomalies, current evidence indicates that the residual chance of structural anomalies and abnormal neurodevelopment is not higher than expected in the general population [6,9].
In our survey including KSUOG members, when fetuses with increased NT have normal karyotype and normal findings of detailed fetal anatomy, 51.6% of respondents (100/194) reported that they explain to parents that the prognosis is good and the pediatric outlook is the same as in the general population. On the other hand, 48.4% of respondents (94/194) said that they tell parents that a chance remains that the pediatric outlook may be worse than expected in the general population.
Fetal cystic hygroma is a congenital malformation of the lymphatic system, typically characterized by edema and a fluid-filled space in the fetal neck [16]. When a cystic hygroma appears septated, the prognosis is worse than when the non-septated form appears [16]. Fetal cystic hygroma is associated with both fetal aneuploidy and major structural abnormalities, including cardiac, skeletal, and pulmonary abnormalities, and with a high risk of miscarriage and disorders such as Noonan's syndrome [16,17]. When a septated cystic hygroma is detected, fetal karyotyping by chorionic villi sampling may be recommended. After completion of a detailed fetal ultrasound and echocardiography at 18 to 22 weeks of gestation, fetuses with normal findings have a higher chance of a normal pediatric outcome [16]. Although cystic hygroma may be considered a disease entity similar to increased NT [16], several researchers have assumed that increased NT and cystic hygroma are anatomically distinct [17]. In our survey including KSUOG members, 23.7% (40/169) reported that septated cystic hygroma is different from isolated increased NT, and that they would recommend termination of pregnancy without chromosomal study confirmation, whereas 76.3% (129/169) would not distinguish septated cystic hygroma from increased NT.
Recently, the timing of fetal anomaly scan has been moving from the second trimester to the first trimester, probably due to widespread use of the NT measurement and advanced ultrasound technology [18,19]. As yet, first-trimester ultrasonography has limited evidence for universal screening because of the underdeveloped fetal organ systems, the characteristics of some anomalies that are not evident until later in a pregnancy, variations in equipment, and limited availability of skilled examiners [20]. Some anomalies, however, can be easily detected during routine ultrasound in the first trimester. Those anomalies are usually lethal and associated with chromosomal abnormalities. This lecture focused on ultrasonography findings and clinical management of several anomalies that can easily be identified during NT measurement at 11-13+6 weeks of gestation.
Anomalies of the head and neck showed the highest detection rate in the first trimester, ranging from 67% to 100% [18]. The acrania-anencephaly sequence, one of the most common lethal anomalies, is a defect of the cranial vault that can be recognized by abnormal head shape and the absence of a round calvarium. It is almost never missed when examining the fetal head on both the mid-sagittal and biparietal diameter planes [21]. Holoprosencephaly is a heterogeneous entity characterized by varying degrees of incomplete separation in the cerebral hemispheres and facial anomalies. Of them, the alobar type can be detected in the first trimester. The biparietal diameter plane is useful for diagnosis by confirming the absence of the characteristic 'butterfly' sign of the choroid plexus (Fig. 2) [22]. Although some studies suggest that fetal karyotyping be offered to parents when alobar holoprosencephaly is detected in first trimester [23], most parents decide to terminate the pregnancy regardless of fetal karyotype because holoprosencephaly is fatal.
Abdominal wall defects, including omphalocele, gastroschisis, and ectopia cordis, are also easily detected on the midsagittal plane of a fetus in the first trimester. Diagnosis of omphalocele should be made carefully after 12 weeks gestation to differentiate a normal physiologic mid-gut hernia (Fig. 3). Once an omphalocele has been detected prenatally, karyotyping should be offered because associated fetal chromosomal abnormalities are common [24]. In contrast to omphalocele, gastroschisis is rarely associated with chromosomal abnormalities; therefore, karyotyping is not considered routinely but only based on individual risk. Ectopia cordis can be diagnosed when a beating heart is located outside the thoracic cage, but only if the apex of the heart is extrathoracic, conclusive diagnosis is difficult in the first trimester. Omphalocele and gastroschisis usually show favorable postnatal outcomes in fetuses with an isolated defect [25,26], but patients with ectopia cordis, most of whom also have intra- and extracardiac defects, rarely survive [27].
Megacystis is defined as an enlarged bladder with a longitudinal diameter greater than 7 mm or 10% of crown rump length [28]. Once the cystic structure is visible on the fetal abdomen, color Doppler should be applied in order to confirm the bladder based on the detection of umbilical arteries by the side. When megacystis is diagnosed prenatally, karyotyping should be performed, and serial follow-ups are needed to detect progressive changes [29]. A significant number of cases with mild or moderate megacystis (a longitudinal diameter less than 15 mm) resolve spontaneously, so expectant management with frequent ultrasonography follow-up is reasonable. However, with progressive changes or a bladder diameter larger than 15 mm, immediate intervention might be required [29,30]. Although repetitive fetal therapies can be performed, perinatal prognosis is usually poor because of associated anomalies [31].
Measurement of the cardiac axis, the angle between the ventricular septum and the midline of the chest on the four-chamber view, is possible in about two-thirds of first trimester scans [32,33]. According to the study of McBrien et al. [34], the mean cardiac axis was 40.5° from 10 to 11+6 weeks, 49° from 12 to 12+6 weeks, 50.6° from 13 to 13+6 weeks, and relatively constant at 45° in the second trimester. In cases of severe congenital heart disease, the cardiac axis might be deviated to the right or left in the first trimester, which can assist in prenatal detection of congenital heart malformations in the second trimester (Fig. 4).
Limb anomalies, such as congenital arthrogryposis and radial aplasia, have also been reported using first-trimester ultrasonography, with remarkable detection rates [18]. Arthrogryposis is described as multiple congenital contractures of two or more joints. The detection rate in the first trimester is less than 50% because contractures can develop later in a pregnancy due to an abnormal uterine environment that restricts fetal movement, such as oligohydramnios. Radial aplasia can be diagnosed when a single forearm bone with radial deviation of the hand is seen in ultrasonography. When arthrogryposis or radial aplasia is diagnosed prenatally, chromosomal analysis should be considered [35,36]. Postnatal outcomes vary depending on their etiologies, but they are generally poor because of associated anomalies.
In conclusion, some anomalies will be identified during the first trimester, and early detection of anomalies can allow parents to make earlier decisions on further management. Ultrasonography examination in the first trimester is best carried out using a standardized protocol to check for these anomalies as well as to perform NT measurement.
This group discussion focused on four important topics regarding ultrasound examination performance, practice, and counseling during the first trimester: 1) Is prenatal ultrasonography safe during the first trimester? 2) Which cutoff should be used for increased NT, and how should parents be counseled? 3) What is a good definition of cystic hygroma? 4) Does the presence of a nasal bone have any diagnostic value for Down syndrome in the Korean population? Given that the medical environment, including ultrasound facilities, varies widely and is influenced by individual circumstances, it is important to understand that these communications are not intended to restrict any doctor's particular practice with careful consideration.
Moderator: Soo-Young Oh, MD, PhD
Panelists: Moon Young Kim, MD, PhD · Joong Shin Park, MD, PhD · Min Jeong Oh, MD, PhD · Mi Hye Park, MD, PhD
The moderator first mentioned the results from the survey including KSUOG members, which showed that up to 95% of respondents used pulsed wave (PW) Doppler to measure the fetal heart rate during the first trimester and then asked the panelists for their opinion regarding ultrasound safety, especially during the first trimester. Dr. JS Park began by reminding the audience of the as low as reasonably achievable (ALARA) principle [37] and the Food and Drug Administration's caution against using prenatal ultrasound for non-medical reasons [38]. He said that it is better to avoid PW Doppler to measure fetal heart rate during the first trimester, if possible. Dr. MJ Oh similarly emphasized that, although no evidence suggests that ultrasound for medical indication harms the fetus, it is prudent to limit PW Doppler to cases in which the fetal heart rate cannot be visualized by the naked eye. Dr. MH Park noted that the time elapsed during an ultrasound scan may be a safety issue and should be regarded as important if detailed anomaly scanning is undertaken during the first trimester. Lastly, Dr. MY Kim mentioned the importance of the thermal index (TI) and introduced the recommendation by the British Medical Ultrasound Society, revised in 2009 (Table 2) [39]. She emphasized the importance of being familiar with the TI and noted that, if TI <0.7, the time limit for obstetric ultrasound can be generous enough to obtain an adequate image, although ALARA principles should be still kept in mind.
This discussion concluded with an agreement that it is necessary for KSUOG to continue to educate physicians performing prenatal ultrasound about the TI, especially for scans in the first trimester. It is clinically sufficient to verify the fetal heart rate by eye. If an abnormal fetal heart rate is suspected, M-mode rather than PW Doppler is preferred. Participants also agreed that the use of PW Doppler in the first trimester is best restricted to cases in which the fetal heart rate cannot be identified by other method.
First, each panelist explained the practice in his or her institution and explained the rationale for the cutoff values used. Dr. MY Kim explained that Cheil Hospital uses the 95th percentile as a cutoff for increased NT, but recommendations for chorionic villi sampling vary depending on physician preference. Dr. MJ Oh also uses the 95th percentile cutoff, but she does not routinely recommend chorionic villi sampling with this cut-off. She noted that the 3 mm cutoff is used as the cutoff for recommending an invasive test. Between the 95th percentile and 3 mm, she offers individualized counseling depending on other risk factors, such as PAPP-A. Dr. MH Park gave an opinion similar to that of Dr. MJ Oh and added that she used the results from a serum screening test (dual test) to determine whether to perform the invasive test to identify abnormal chromosomes. Dr. JS Park said that he used the 3 mm cutoff for increased NT and explained that the detection rate is higher when using the 95th percentile as a cutoff, but that the false positive rate may also be higher. Dr. JS Park emphasized that the final decision to choose an invasive test should be made by patients, not by doctors. The 3.5 mm was seldom used by experts as a cutoff for increased NT.
First-trimester cystic hygroma is a developmental anomaly of the lymphatic system characterized by a fluid-filled space at sites of the lymphatic-venous connection within the posterior neck and back of a fetus [40]. However, as the definition of the cystic hygroma is vague, clinicians are frequently uncertain whether to designate the finding 'increased NT' or 'cystic hygroma.' In this session, panelists noted the definition of cystic hygroma used in their practices. Dr. MH Park explained that she differentiated cystic hygroma from increased NT when septation within the fluid-filled space was observed. However, she pointed out that the management of either cystic hygroma or increased NT depends on the thickness of the fluid-filled space. Dr. JS Park and Dr. MJ Oh explained that they diagnosed cystic hygroma when the fluid-filled space extended to the entire body of fetus along with septation. Dr. MY Kim added that the enlarged jugular sac can be used to differentiate these two afflictions, as described by Ville [41]. Panelists agreed that cystic hygroma has poorer prognosis than increased NT, as presented by other reports indicating that cystic hygroma was associated with abnormal chromosomes 54.9% of the time and a major malformation 28.8% of the time [17]. However, they acknowledged that contemporary management of cystic hygroma and increased NT are practically the same.
Considering that, in some clinical situations, pregnancy termination was recommended without an invasive test or close follow-up after a diagnosis of cystic hygroma, the panelists recommended that the diagnosis of cystic hygroma needed to be limited to cases when the fluid-filled space extended to the entire body of the fetus with concomitant visualization of septation. They added that such a strict definition could help to prevent cases of unnecessary pregnancy termination.
Because evidence in the Western countries has suggested that the absence of a nasal bone in the first trimester is associated with increased risk of Down syndrome, nasal bone assessment is becoming part of fetal aneuploidy screening in Korea [42,43]. However, considering the relatively higher rate of small nasal bones in Asians [44], it was considered important to discuss and share practice patterns on this topic. All panelists recognized that, unlike increased NT, the absence of a nasal bone alone in the first trimester has not been proven useful as an aneuploidy screening through any prospective randomized studies. In addition, Dr. MH Park emphasized that it is not always easy to assess the nasal bone. A considerable amount of experience is required to attain enough proficiency to see the nasal bone in every case, as indicated previously [45]. She emphasized that it is prudent to schedule a follow-up scan 1 or 2 weeks later if the nasal bone is not clearly visible in the first trimester. She also noted that the absence of a nasal bone becomes more meaningful when increased NT or abnormalities of the ductus venosus are concurrently observed. In addition, it was stressed to use "non-visualization of the nasal bone" rather than "absence of the nasal bone" when describing ultrasonography findings.
No panelist recommended an invasive test for fetal chromosomes when absence of the nasal bone was observed as an isolated sonographic abnormality. The panelists concluded that it might be unnecessary to inform pregnant women about non-visualization of the nasal bone in the absence of other abnormal findings in a detailed ultrasound examination.
Figures and Tables
 | Fig. 2(A) Ultrasonography of a normal fetus at 12 weeks of gestation shows the butterfly sign of the choroid plexus on the biparietal diameter plane. (B) A fetus with holoprosencephaly at 13 weeks of gestation. The butterfly sign is absent, and a single ventricle is present on the biparietal diameter plane. |
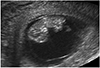 | Fig. 3Sagittal view of a normal fetus at 10 weeks of gestation shows normal physiologic hernia on abdomen. This hernia sac has disappeared after 12 weeks of gestation. |
References
1. Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992; 304:867–869.
2. Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation: Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998; 352:343–346.
3. Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003; 10:56–104.
4. Nicolaides KH, Spencer K, Avgidou K, Faiola S, Falcon O. Multicenter study of first-trimester screening for trisomy 21 in 75 821 pregnancies: results and estimation of the potential impact of individual risk-orientated two-stage first-trimester screening. Ultrasound Obstet Gynecol. 2005; 25:221–226.
5. Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ. 1988; 297:883–887.
6. Souka AP, Von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH. Increased nuchal translucency with normal karyotype. Am J Obstet Gynecol. 2005; 192:1005–1021.
7. Hyett J, Perdu M, Sharland G, Snijders R, Nicolaides KH. Using fetal nuchal translucency to screen for major congenital cardiac defects at 10-14 weeks of gestation: population based cohort study. BMJ. 1999; 318:81–85.
8. Kim MH, Park SH, Cho HJ, Choi JS, Kim JO, Ahn HK, et al. Threshold of nuchal translucency for the detection of chromosomal aberration: comparison of different cut-offs. J Korean Med Sci. 2006; 21:11–14.
9. Souka AP, Snijders RJ, Novakov A, Soares W, Nicolaides KH. Defects and syndromes in chromosomally normal fetuses with increased nuchal translucency thickness at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1998; 11:391–400.
10. Nicolaides KH. First-trimester screening for chromosomal abnormalities. Semin Perinatol. 2005; 29:190–194.
11. Pandya PP, Kondylios A, Hilbert L, Snijders RJ, Nicolaides KH. Chromosomal defects and outcome in 1015 fetuses with increased nuchal translucency. Ultrasound Obstet Gynecol. 1995; 5:15–19.
12. Mol BW. Down's syndrome, cardiac anomalies, and nuchal translucency. BMJ. 1999; 318:70–71.
13. Ayras O, Tikkanen M, Eronen M, Paavonen J, Stefanovic V. Increased nuchal translucency and pregnancy outcome: a retrospective study of 1063 consecutive singleton pregnancies in a single referral institution. Prenat Diagn. 2013; 33:856–862.
14. Bellotti M, Fesslova V, De Gasperi C, Rognoni G, Bee V, Zucca I, et al. Reliability of the first-trimester cardiac scan by ultrasound-trained obstetricians with high-frequency transabdominal probes in fetuses with increased nuchal translucency. Ultrasound Obstet Gynecol. 2010; 36:272–278.
15. Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol. 2004; 191:45–67.
16. Malone FD, Ball RH, Nyberg DA, Comstock CH, Saade GR, Berkowitz RL, et al. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005; 106:288–294.
17. Scholl J, Durfee SM, Russell MA, Heard AJ, Iyer C, Alammari R, et al. First-trimester cystic hygroma: relationship of nuchal translucency thickness and outcomes. Obstet Gynecol. 2012; 120:551–559.
18. Rossi AC, Prefumo F. Accuracy of ultrasonography at 11-14 weeks of gestation for detection of fetal structural anomalies: a systematic review. Obstet Gynecol. 2013; 122:1160–1167.
19. Katorza E, Achiron R. Early pregnancy scanning for fetal anomalies: the new standard? Clin Obstet Gynecol. 2012; 55:199–216.
20. Kontopoulos E, Odibo A, Wilson RD. Current controversies in prenatal diagnosis 2: are we ready to screen for fetal anomalies with first trimester ultrasound? Prenat Diagn. 2013; 33:9–12.
21. Syngelaki A, Chelemen T, Dagklis T, Allan L, Nicolaides KH. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11-13 weeks. Prenat Diagn. 2011; 31:90–102.
22. Sepulveda W, Wong AE. First trimester screening for holoprosencephaly with choroid plexus morphology ('butterfly' sign) and biparietal diameter. Prenat Diagn. 2013; 33:1233–1237.
23. Kagan KO, Staboulidou I, Syngelaki A, Cruz J, Nicolaides KH. The 11-13-week scan: diagnosis and outcome of holoprosencephaly, exomphalos and megacystis. Ultrasound Obstet Gynecol. 2010; 36:10–14.
24. Khalil A, Arnaoutoglou C, Pacilli M, Szabo A, David AL, Pandya P. Outcome of fetal exomphalos diagnosed at 11-14 weeks of gestation. Ultrasound Obstet Gynecol. 2012; 39:401–406.
25. Tassin M, Descriaud C, Elie C, Houfflin Debarge V, Dumez Y, Perrotin F, et al. Omphalocele in the first trimester: prediction of perinatal outcome. Prenat Diagn. 2013; 33:497–501.
26. David AL, Tan A, Curry J. Gastroschisis: sonographic diagnosis, associations, management and outcome. Prenat Diagn. 2008; 28:633–644.
27. Amato JJ, Douglas WI, Desai U, Burke S. Ectopia cordis. Chest Surg Clin N Am. 2000; 10:297–316.
28. Sebire NJ, Von Kaisenberg C, Rubio C, Snijders RJ, Nicolaides KH. Fetal megacystis at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1996; 8:387–390.
29. Liao AW, Sebire NJ, Geerts L, Cicero S, Nicolaides KH. Megacystis at 10-14 weeks of gestation: chromosomal defects and outcome according to bladder length. Ultrasound Obstet Gynecol. 2003; 21:338–341.
30. Sepulveda W. Megacystis in the first trimester. Prenat Diagn. 2004; 24:144–149.
31. Al-Hazmi H, Dreux S, Delezoide AL, Dommergues M, Lortat-Jacob S, Oury JF, et al. Outcome of prenatally detected bilateral higher urinary tract obstruction or megacystis: sex-related study on a series of 709 cases. Prenat Diagn. 2012; 32:649–654.
32. Comstock CH. Normal fetal heart axis and position. Obstet Gynecol. 1987; 70:255–259.
33. Souka AP, Pilalis A, Kavalakis Y, Kosmas Y, Antsaklis P, Antsaklis A. Assessment of fetal anatomy at the 11-14-week ultrasound examination. Ultrasound Obstet Gynecol. 2004; 24:730–734.
34. McBrien A, Howley L, Yamamoto Y, Hutchinson D, Hirose A, Sekar P, et al. Changes in fetal cardiac axis between 8 and 15 weeks' gestation. Ultrasound Obstet Gynecol. 2013; 42:653–658.
35. Reed SD, Hall JG, Riccardi VM, Aylsworth A, Timmons C. Chromosomal abnormalities associated with congenital contractures (arthrogryposis). Clin Genet. 1985; 27:353–372.
36. Sepulveda W, Treadwell MC, Fisk NM. Prenatal detection of preaxial upper limb reduction in trisomy 18. Obstet Gynecol. 1995; 85(5 Pt 2):847–850.
37. Nelson TR, Fowlkes JB, Abramowicz JS, Church CC. Ultrasound biosafety considerations for the practicing sonographer and sonologist. J Ultrasound Med. 2009; 28:139–150.
38. US Food and Drug Administration. Fetal keepsake videos [Internet]. Silver Spring: US Food and Drug Administration;cited 2014 Oct 8. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PatientAlerts/ucm064756.htmhttp://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PatientAlerts/ucm064756.htm.
39. The British Medical Ultrasound Society. Guidelines for the safe use of diagnostic ultrasound equipment [Internet]. London: The British Medical Ultrasound Society;cited 2014 Oct 8. Available from: http://fetalanomaly.screening.nhs.uk/fetalanomalyresource/images/stories/Downloads/2.5.1_safe_environment/bmus-safety-guidelines-2009-revision-final-nov-2009.pdf.
40. Johnson MP, Johnson A, Holzgreve W, Isada NB, Wapner RJ, Treadwell MC, et al. First-trimester simple hygroma: cause and outcome. Am J Obstet Gynecol. 1993; 168(1 Pt 1):156–161.
41. Ville Y. Nuchal translucency in the first trimester of pregnancy: ten years on and still a pain in the neck? Ultrasound Obstet Gynecol. 2001; 18:5–8.
42. Cicero S, Avgidou K, Rembouskos G, Kagan KO, Nicolaides KH. Nasal bone in first-trimester screening for trisomy 21. Am J Obstet Gynecol. 2006; 195:109–114.
43. Has R, Kalelioglu I, Yuksel A, Ibrahimoglu L, Ermis H, Yildirim A. Fetal nasal bone assessment in first trimester down syndrome screening. Fetal Diagn Ther. 2008; 24:61–66.
44. Moon MH, Cho JY, Lee YM, Lee YH, Yang JH, Kim MY, et al. Nasal bone length at 11-14 weeks of pregnancy in the Korean population. Prenat Diagn. 2006; 26:524–527.
45. Malone FD, Ball RH, Nyberg DA, Comstock CH, Saade G, Berkowitz RL, et al. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004; 104:1222–1228.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download