Abstract
Congenital cystic adenomatoid malformation (CCAM) is a rare condition which is easily detectable by prenatal ultrasonography. Fetuses with large CCAMs associating with hydrops are predisposed to perinatal mortality, therefore prenatal intervention is required. While macrocystic CCAM is treated prenatally by thoracentesis or thoraco-amniotic shunt, microcystic or mixed CCAM is difficult to manage in the fetus. In these latter lesions, fetal lobectomy, sclerotherapy, or laser ablation was used to treat lesions directly. We present an unusual prenatal case of mixed CCAM associating with hydrops and marked ascites, which was conservatively managed with prenatal abdomino-amniotic shunting and successfully treated by postnatal surgery.
Congenital cystic adenomatoid malformation (CCAM) is a rare condition resulting from an overgrowth of the terminal bronchioles [1]. Because large CCAMs can be associated with polyhydramnios, fetal hydrops, and subsequent fetal death [1], prenatal intervention is crucial to improve perinatal outcome. In cases of macrocystic CCAM, decompression can be achieved by thoracentesis or thoraco-amniotic shunt, while fetal lobectomy, sclerotherapy, or laser ablation is optional treatment to reduce the mass size and subsequently induce lung growth in cases of microcystic or mixed CCAM [2]. While these modalities treat lesions directly, there is no report on the minimal intervention of large CCAMs, which cannot be treated prenatally. In this report, we describe a successful intrauterine palliative treatment with abdomino-amniotic shunting of a large CCAM associating with hydrops and marked ascites, followed by postnatal resection of the mass.
A 33-year-old primi gravida was referred to our tertiary center at 21+3 weeks of gestation because of a suspected large thoracic mass with ascites. Ultrasonography was performed using the Accuvix A30 (Samsung Medison Co. Ltd., Seoul, Korea) with a 2 to 6 MHz transabdominal probe. The initial ultrasonography revealed a huge hyperechoic and well-defined mass in the right lung, occupying approximately three-quarters of the thoracic volume (Fig. 1A). A cystic lesion of 25 mm in diameter was located centrally within the mass and this mass was supplied by the pulmonary artery (Fig. 1B). The fetus was diagnosed with mixed CCAM. The heart was forced to the left side of the thoracic cavity, which made the left lung nearly invisible on the ultrasonography. The fetus was hydrophic, with massive ascites and scalp edema, and left cardiac function was decreased. No other abnormalities were found.
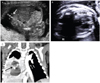
Following extensive counseling on the prognosis and the possibility of intrauterine death, the parents chose to continue the pregnancy. The parents also did not wish to perform fetal karyotyping. Because of massive ascites, we decided to perform the abdomino-amniotic shunting immediately. The patient was admitted at 21+3 weeks of gestation and abdomino-amniotic shunting was performed. After successful shunting, the ascites disappeared (Fig. 2A) and the fetal hydrops was also resolved two weeks following the shunting. Serial ultrasonographs showed an alleviation of the mediastinal shifting, a decrease in mass size, and the appearance of the left lung (Fig. 2B). Cardiac function also improved.
A follow-up ultrasound at 36+1 weeks of gestation demonstrated retarded fetal growth with oligohydramnios, and therefore we decided to deliver the baby. The mother delivered an 1,890 g female fetus by Cesarean section at 36+3 weeks of gestation. Initial crying was good and the Apgar score were 7 and 8 at 1 and 5 minutes, respectively. However the baby developed respiratory distress soon after birth and was placed on a mechanical ventilator. Chest computed tomography (CT) showed a large solid mass with an internal cystic lesion in the right middle to lower lung, suggestive of CCAM (Fig. 2C). On postnatal day 5, a lobectomy of the right middle and lower lung was performed. A pathology report confirmed the diagnosis of mixed CCAM. The baby was doing well without any respiratory symptoms at one month of follow-up.
To our knowledge, this is the first report to describe the successful treatment of a large CCAM with hydrops by a conservative approach that involves minimal intervention. All reported cases of intrauterine treatment in hydrophic fetuses with large CCAMs were conceived mainly to reduce the size of the mass. There are several prenatal treatment options that are based on the characteristics of lesions. Macrocystic CCAM can be treated by decompression of the cystic mass performing thoracentesis or thoraco-amniotic shunt, whereas microcystic or mixed CCAM is more difficult to manage prenatally. In these latter types of lesions, additional interventions, such as open fetal lobectomy, which is the therapy of choice, have been attempted [2]. Adzick [3] recently reported a survival rate of 54% (13 survivors out of 24 cases with large CCAMs) following open fetal lobectomy. Although fetuses with life threatening lung lesions can survive with this aggressive treatment, there is also significant morbidity and mortality. Moreover it have been performed in only few experienced centers, and therefore is limited to perform in several other centers. Although other less invasive procedures such as percutaneous interstitial ablation or injection of a sclerosing agent into the CCAM have also been performed, few successful cases were reported so far [4,5]. Therefore, additional studies are needed to identify the best treatment approach for fetuses with large CCAMs. In this report, we describe a successful intrauterine palliative treatment with abdomino-amniotic shunting in a fetus with a large CCAM and hydrops. On the basis of these results, we suggest that conservative minimal intervention is an additional approach to treat a large CCAM associating with hydrops, which cannot be treated prenatally.
Large lung lesions can result in polyhydramnios because of esophageal compression by the mass, causing difficulties with swallowing. Large lesions can also induce fetal hydrops by obstructing the vena cava and compressing the heart [1]. Fetuses without hydrops show good perinatal outcome, whereas fetuses with hydrops are at a higher risk for perinatal death. Therefore, prenatal intervention is warranted in hydropic fetuses [1].
Maternal steroid administration has been attempted in fetuses with microcystic CCAM and hydrops [6-8], and additional larger studies demonstrated the positive effect of this noninvasive treatment [9,10]. Although the exact mechanism by which steroid administration can resolve hydrops and improve an infant's survival rate is still unclear, the potential mechanism is either by decreasing the mass size or by increasing the maturational status of the mass [9]. A standard regimen of prenatal betamethasone, 12 mg intramuscularly, with two doses administrated 24 hours apart, is given to the mothers after making the diagnosis with large microcystic CCAM of their fetuses, and usually during the second trimester. However, as in several reported cases there was a variable response to steroid administration [9-11], a prospective trial will be needed in order to confirm the effect of steroid. In our case, steroid therapy was not considered for the first-line treatment because the most successful method was required for decompression of massive ascites.
Large CCAMs detected by ultrasonography may decrease in size or regress spontaneously during fetal life [12]. In our case, the large mass occupied nearly the entire thoracic cavity, and the left lung was not observed during an initial examination. Massive ascites might induce further obstruction of the venous drainage and compression of the heart, thus aggravating the fetal hydrops. However, after decompression of the abdominal pressure by removing the ascites, the hydrops was resolved and the lung became visible on ultrasound at approximately 30 weeks of gestation. Postnatal surgery confirmed that the lesion was localized in only the right middle to lower lung. Therefore, parents of a fetus diagnosed with a large CCAM should be counseled about the natural course of CCAM and should be encouraged to continue the pregnancy, even if the CCAM associates with hydrops.
In conclusion, we describe a successful conservative treatment with minimal intervention for a large CCAM with marked ascites. From our case, we suggest that not only the active treatment, but also the conservative management during the prenatal period can save the fetus with a large CCAM and hydrops.
Figures and Tables
Fig. 1
(A) Ultrasonography showing a hyperechoic mass of 70 mm with an enclosed cystic lesion of 25 mm in the right lung with massive ascites surrounding the liver. (B) The mass is supplied by the pulmonary artery (arrow), and the heart is severely deviated to the left side of the thoracic cavity. Lt, left; Rt, right.
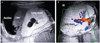
Fig. 2
(A) Placement of the abdomino-amniotic shunt (arrow) in situ to eliminate ascites. (B) Ultrasonographic findings at 31+2 weeks of gestation showing the left lung with alleviated mediastinal shifting. (C) Postnatal chest computed tomography showing the right lung with a centrally located congenital cystic adenomatoid malformation (arrow) and the left normal lung. Lt, left; Rt, right.
References
1. Adzick NS, Harrison MR, Crombleholme TM, Flake AW, Howell LJ. Fetal lung lesions: management and outcome. Am J Obstet Gynecol. 1998; 179:884–889.
2. Witlox RS, Lopriore E, Oepkes D. Prenatal interventions for fetal lung lesions. Prenat Diagn. 2011; 31:628–636.
3. Adzick NS. Open fetal surgery for life-threatening fetal anomalies. Semin Fetal Neonatal Med. 2010; 15:1–8.
4. Bermudez C, Perez-Wulff J, Arcadipane M, Bufalino G, Gomez L, Flores L, et al. Percutaneous fetal sclerotherapy for congenital cystic adenomatoid malformation of the lung. Fetal Diagn Ther. 2008; 24:237–240.
5. Ruano R, da Silva MM, Salustiano EM, Kilby MD, Tannuri U, Zugaib M. Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenat Diagn. 2012; 32:1127–1132.
6. Higby K, Melendez BA, Heiman HS. Spontaneous resolution of nonimmune hydrops in a fetus with a cystic adenomatoid malformation. J Perinatol. 1998; 18:308–310.
7. Tsao K, Hawgood S, Vu L, Hirose S, Sydorak R, Albanese CT, et al. Resolution of hydrops fetalis in congenital cystic adenomatoid malformation after prenatal steroid therapy. J Pediatr Surg. 2003; 38:508–510.
8. Leung WC, Ngai C, Lam TP, Chan KL, Lao TT, Tang MH. Unexpected intrauterine death following resolution of hydrops fetalis after betamethasone treatment in a fetus with a large cystic adenomatoid malformation of the lung. Ultrasound Obstet Gynecol. 2005; 25:610–612.
9. Peranteau WH, Wilson RD, Liechty KW, Johnson MP, Bebbington MW, Hedrick HL, et al. Effect of maternal betamethasone administration on prenatal congenital cystic adenomatoid malformation growth and fetal survival. Fetal Diagn Ther. 2007; 22:365–371.
10. Curran PF, Jelin EB, Rand L, Hirose S, Feldstein VA, Goldstein RB, et al. Prenatal steroids for microcystic congenital cystic adenomatoid malformations. J Pediatr Surg. 2010; 45:145–150.
11. Morris LM, Lim FY, Livingston JC, Polzin WJ, Crombleholme TM. High-risk fetal congenital pulmonary airway malformations have a variable response to steroids. J Pediatr Surg. 2009; 44:60–65.
12. Miller JA, Corteville JE, Langer JC. Congenital cystic adenomatoid malformation in the fetus: natural history and predictors of outcome. J Pediatr Surg. 1996; 31:805–808.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download