Abstract
In the conservative management of uterine fibroids is radiofrequency ablation (RFA) considered to be one of the safe, effective and minimal invasive approaches in selected women who desire to retain their uterus. Few studies were conducted on its adverse outcomes and most of the reported complications were minor events such as pain, discharge, adhesion which didn't require any intervention. However, although safe and effective, the RFA of a uterine myoma can be the cause for severe complications such as penetration and burn injuries of pelvic organs. In general, a rectouterine fistula is one of the rarest complications but can lead to serious adverse outcomes. Herein, to our knowledge, we report the first case involving a rectouterine fistula after laparoscopic ultrasound-guided RFA of a uterine myoma with pelvic endometriosis. In addition, we provide a brief review of the relevant literature.
Recently, many studies reported that radiofrequency ablation (RFA) is considered to be a safe, effective and minimal invasive approach with low complication rates and minimal patient discomfort in the conservative management of uterine fibroids in selected woman who desire to retain their uterus [1,2,3].
However, few studies were conducted regarding the adverse outcomes and the most reported complications are minor events such as pain, discharge and adhesion without need for further intervention. Penetration and burn injuries of the bowel or bladder have never been reported related with RFA of uterine fibroids. Although it is safe and effective, several reports showed that RFA can give rise to severe complications in other organs such as bronchopleural, bronchobiliary or renoduodenal fistula [4,5,6]. An enterouterine fistula occurs extremely rare, some cases have been reported referring to Crohn's disease or diverticulitis where it was difficult to save the uterus and fertility [7,8,9,10]. As in our case, a rectouterine fistula may occur during RFA of a uterine fibroid. Therefore, thorough counseling of the potential benefits and risks is essential prior to the procedure and the possibility of conductive thermal injury to the adjacent organs should be borne in mind by the surgeon.
We present herein, to our knowledge, the first case involving a rectouterine fistula after laparoscopic ultrasound-guided RFA of a uterine fibroid with pelvic endometriosis.
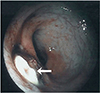
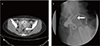
A 36-year-old, nulligravida, unwanted for pregnancy was referred to our hospital due to persistent dysmenorrhea and lower back pain following a bilateral uterine artery embolization and intrauterine device (Mirena) insertion at a local clinic. A pelvic magnetic resonance imaging showed a 5-cm-heterogeneous mass on the posterior uterine wall, which appeared to be an intramural fibroid. After thorough counseling on the potential risks and benefits of the procedure and the possible alternative surgical treatments was the laparoscopic ultrasound-guided RFA performed under general anesthesia. A dense adhesion was found in the right cul-de-sac between uterus and colon. Adhesiolysis and biopsies were performed of the adhesive band and peritoneum and an endometriosis was later confirmed. A 16-gauge RFA needle was percutaneously inserted and placed within the target fibroid under laparoscopic video and transvaginal ultrasound guidance. The RF delivery system (M1004, RF Medical Co., Seoul, Korea) was applied twice with a maximum power of 120 watt and a maximum temperature of 85℃ for 5 minutes. The patient was discharged without any complications. However, she visited the hospital for bloody anal discharge and vaginal leakage of stool after two weeks of discharge. During her visit were the vital signs stable, but a leukocytosis (white blood cell 12,820/µL) and an elevation of C-reactive protein (1.4 mg/dL) were observed. The colonoscopy showed a white, marginal protruding mucosal defect about 9 cm above the anal verge (Fig. 1). A pelvic computed tomography and a hysterosalpingogram revealed a fistula between the posterior uterine wall and the rectum (Fig. 2). The patient underwent a rectal fistula wedge resection and ileostomy and total abdominal hysterectomy due to severe inflammation and necrosis in the uterine cavity. Two months later, the fluoroscopic examination of the pouchgram showed an improved rectouterine fistula and no leakage of contrast media, thus a take-down of the ileostomy was performed. There was no further clinical problem afterward for one year of follow-up.
RFA has become a widespread technique for achieving local control of tumors in various organs which are no resection candidates involving the liver, kidney and lungs. Especially, in the conservative management of uterine fibroids is RFA considered to be one of the safe, effective and minimal invasive approaches for women who desire to retain their uterus [1,2,3]. Recent studies showed about 65% to 80% of myoma volume reduction 6 months after the procedure. The symptoms improvement rate was about the same [1,2].
Complications after RFA are rare, but Kim et al. [2] presented lower abdominal pain and vaginal discharge that improved within 2 weeks and Garza et al. [1] also described abdominal pain, urinary tract infection and abdominal vascular injury which were solved without any problem. However, severe thermal injuries and complications can occur during RFA of uterine fibroids. There was an unusual case where a total abdominal hysterectomy was performed due to a uterine abscess which was formed after myolysis [11]. Also there are several case reports on severe complications after RFA in other organs, especially fistulas, such as bronchopleural, bronchobiliary and renoduodenal which were treated by massive antibiotics or surgical resection [4,5,6].
Generally, fistulous formation rarely occurs between the uterus and intestinal tract and it is thought to be related to the great thickness of the uterine wall. The causes implicated are traumatic or spontaneous rupture of a gravid uterus, perforation of the uterine wall and bowel with an intrauterine device, inflammatory diseases or malignancy of pelvic organs [7,8,9,10]. Patchell et al. [7] reported a rectouterine fistula after the malpositioned Cu-7 intrauterine contraceptive device with spontaneous closure. Stoddard et al. [8] presented a patient with a rectouterine fistula in Crohn's disease after the performance of a transverse colostomy without hysterectomy. Dias et al. [10] also reported a patient with ileouterine fistula in Crohn's disease, which was corrected by an ileocecal resection and surgical repair of the uterus. Sentilhes et al. [9] reported a colouterine fistula, secondary to diverticulitis, which was treated with an en bloc resection of the uterus and sigmoid colon. In the treatment of an enterouterine fistula, an active surgical treatment is usually indicated depending upon the etiology, the patient's age and the state of lesions, etc. So, each case must be individualized as soon as it is encountered.
During a RFA, the fibroid should be carefully monitored by ultrasonography and the duration of ablation should be determined based on the increased echogenicity of the fibroid to minimize a thermal injury due a RFA. The progression of ablation is considered to be good if a postechogenic shadow is observed. The ablation should be discontinued when the fibroid exhibits high echogenicity during ablation and the area of high echogenicity reaches 90% of the fibroid. Generally, an increased echogenicity disappears 20 minutes after ablation. The complete ablation of a 3-cm-large myoma usually takes 5 minutes, while that of an approximately 5-cm-sized myoma takes almost 10 minutes. Multiple overlapping ablation cycles to obtain complete ablation should be carefully performed for fibroids with a mean diameter greater than 5 cm [12].
In our thought, a uterus related fistula may generally less occur due to the thickness of the myometrium. However, a fistula in adjacent organs can occur during RFA of a uterine fibroid by thermal damage if safe procedure guidelines such as depth of needle insertion, duration of lysis and monitoring by ultrasonography are not kept.
Recently, several studies proved the safety and effectiveness of RFA for uterine fibroids either by a laparoscopic transabdominal or transvaginal approach. However, there is no comparative study between the laparoscopic transabdominal and transvaginal approach. In our opinion, the laparoscopic transabdominal approach would minimize the thermal damage in cases of suspected severe adhesions or co-existing endometriosis.
Before the RFA of uterine fibroids should be considered myoma-related symptoms, previous medical treatment, number of myoma and the plan for future pregnancies [2,3]. Generally, RFA is not recommended for cases of huge multiple myoma, current pregnancy, suspected malignancy, abnormal coagulation test, recent pelvic inflammatory disease and so on [1,2,3]. Up to now, there has been no complete consensus on its indications and contraindications.
No safety on RFA in uterine fibroids has established yet in women with future planned pregnancy. Several adverse outcomes related to subsequent pregnancies, such as uterine rupture were reported after RFA [13,14]. However, Phillips et al. [15] reported cases of uncomplicated full-term pregnancies indicating that viable pregnancies were possible after laparoscopic myolysis and Kim et al. [2] reported three patients that experienced successful conception and childbirth after RF myolysis procedure. Therefore, it is important to carefully select patients for RFA, considering the number, size and location of the myoma as well as the age and pregnancy plans of the patients.
In summary, a rectouterine fistula is very rare, but it can possibly occur during the RFA of a uterine fibroid. In such cases, it may be difficult to conduct the conservative management preserving the uterus or fertility due to severe inflammation or necrosis of the endometrium. Therefore, thorough counseling of the potential benefits and risks is essential prior to the procedure and the possibility of conductive thermal injury to the adjacent organs should be borne in mind by the surgeon in cases with suspected severe adhesions or co-existing endometriosis. Herein, to our knowledge, we report the first case involving a rectouterine fistula after laparoscopic ultrasound-guided RFA of a uterine fibroid together with a literature review.
Figures and Tables
References
1. Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 Ablation System. J Minim Invasive Gynecol. 2011; 18:364–371.
2. Kim CH, Kim SR, Lee HA, Kim SH, Chae HD, Kang BM. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011; 26:559–563.
3. Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years' outcome from the Halt trial. Health Qual Life Outcomes. 2013; 11:139.
4. Li W, Huang L, Han Y, Zhou Y, Lu Q, Li X. Bronchopleural fistula after non small cell lung cancer radiofrequency ablation: what it implying to us? Diagn Pathol. 2013; 8:202.
5. Kim YS, Rhim H, Sung JH, Kim SK, Kim Y, Koh BH, et al. Bronchobiliary fistula after radiofrequency thermal ablation of hepatic tumor. J Vasc Interv Radiol. 2005; 16:407–410.
6. De Arruda HO, Goldman S, Andreoni C, Maia RS, Szejnfeld J, Ortiz V. Renoduodenal fistula after renal tumor ablation with radiofrequency. Surg Laparosc Endosc Percutan Tech. 2006; 16:342–343.
7. Patchell RD. Rectouterine fistula associated with the Cu-7 intrauterine contraceptive device. Am J Obstet Gynecol. 1976; 126:292–293.
8. Stoddard CJ, Irvin TT. Rectouterine fistulation in Crohn's disease. Br Med J. 1977; 1:1574–1575.
9. Sentilhes L, Foulatier O, Verspyck E, Roman H, Scotte M, Marpeau L. Colouterine fistula complicating diverticulitis: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003; 110:107–110.
10. Dias VC, Lago P, Santos M. Ileouterine fistula: an unusual complication of Crohn's disease. Inflamm Bowel Dis. 2008; 14:1465–1466.
11. Kim HW, Han KH, Cha DS, Kim TH, Kwon MS, Park EY. Uterine abscess after radiofrequency myolysis of uterine myoma: a case report. Korean J Obstet Gynecol. 2007; 50:1778–1781.
12. Kim JH. Radiofrequency myolysis of uterine myoma. J Womens Med. 2008; 1:1–5.
13. Vilos GA, Daly LJ, Tse BM. Pregnancy outcome after laparoscopic electromyolysis. J Am Assoc Gynecol Laparosc. 1998; 5:289–292.
14. Arcangeli S, Pasquarette MM. Gravid uterine rupture after myolysis. Obstet Gynecol. 1997; 89:857.
15. Phillips DR, Milim SJ, Nathanson HG, Haselkorn JS. Experience with laparoscopic leiomyoma coagulation and concomitant operative hysteroscopy. J Am Assoc Gynecol Laparosc. 1997; 4:425–433.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download