Abstract
Choriocarcinoma is a highly invasive and metastatic neoplasm which arises in women of reproductive age. It can be either gestational or nongestational in origin, but the latter form is very rare. Choriocarcinoma is characterized by the production of human chorionic gonadotropin. It can metastasize to distant organs such as lung, brain, liver, kidney, and vagina in the early stages of disease, but retroperitoneal metastasis is extremely rare. Treatment options include surgical intervention and chemotherapy. We present the case of a 25-year-old nulliparous woman who presented to our department with a retroperitoneal mass and negative urine human chorionic gonadotropin test, who was immunohistopathologically diagnosed with nongestational choriocarcinoma. The patient responded well to surgery and multi-drug chemotherapy.
Choriocarcinoma is the most malignant tumor type among gestational trophoblastic diseases. It is characterized by the production of human chorionic gonadotropin (hCG) and is usually intrauterine and gestational. Nongestational choriocarcinoma is very rare, and diagnosis is difficult especially in patients of reproductive age. Hematogenous metastasis develops in early stages of the disease [1]. The most commonly involved sites are the lungs (80%), vagina (30%), pelvis (20%), liver (10%), and the brain (10%). The retroperitoneal space is an exceptional location [2]. We report a case of retroperitoneal nongestational choriocarcinoma in a 25-year-old woman with negative hCG tests. Our report may provide informative data for the diagnosis and treatment of this rare disease.
A 25-year-old woman, nulligravida, presented to the emergency department complaining of left lower abdominal pain, fever, vomiting, and nausea that had persisted for two days. She had regular menstrual cycles and her last menstrual period was one week prior to presentation. She was using an intrauterine contraceptive device. Urine hCG tests were negative.
Speculum examination revealed a closed cervix. The uterus was anteverted and normal in size. Transvaginal and transabdominal ultrasounds demonstrated a 6×6×4-cm mass in the left adnexal region. The mass encircled the left common and external iliac arteries. The laboratory tests revealed elevated white blood cell count (16.3×109/L) and elevated C-reactive protein level (11.75 mg/dL). Abdominal computed tomography and magnetic resonance imaging showed left hydronephrosis and a heterogenous enhancing mass in the left retroperitoneum that was encircling the left external and internal iliac arteries. Positron emission tomography also demonstrated a suspicious malignant mass in the left iliac region, rectouterine pouch, and left side of the uterus. No other metastatic lesions were found by imaging studies (Fig. 1).
The patient was transferred to cardiovascular surgery with a presumptive diagnosis of angiosarcoma. During laparotomy, a 9×7×5-cm soft, friable retroperitoneal mass was found, which was encircling the left external and internal iliac arteries, left external iliac vein, and ureter. A 1.5×1.5-cm mass was detected on the paracervical surface of the uterus. Both ovaries and Fallopian tubes appeared to be normal. Mass en bloc resection was performed including the left common iliac artery, left external and internal iliac arteries, left external iliac vein, and ureter. Anastomosis was performed between the left common iliac and left external iliac arteries with 8mm ringed polytetrafluoroethylene. The left internal iliac artery branch was ligated. The left external vein was anastomosed with the auto-saphenous vein.
The tumor was immunopositive for CK7, EMA, hCG, P63, and P53, and negative for PANCK, CK20, CD31, CD56, S-100, CD34, desmin, SMA, and vimentin (Fig. 2). Unlike the presumptive clinical impression of angiosarcoma, the immunohistopathologic findings confirmed a diagnosis of retroperitoneal choriocarcinoma, stage IV. Choriocarcinoma was also present in the left external iliac lymph node, left common iliac lymph node, ureter serosa, and paracervical mass. The patient suffered from extensive deep vein thrombosis on postoperative day 6. She underwent thrombolysis therapy and recovered well thereafter. Levels of carcinoembryonic antigen, CA-125, β-hCG, and alpha-fetoprotein were examined two weeks after surgery and found to be within normal ranges except CA-125, which was 106.4 U/mL (normal reference <35 U/mL). β-hCG was 1.8 mIU/mL, which was within the normal range (normal reference <2 mIU/mL). Endometrial biopsy was performed to rule out gestational origin. Histological examination of the endometrium revealed an inactive endometrium.
A chemotherapy regimen of etoposide, high-dose methotrexate with folic acid, actinomycin D, cyclophosphamide, and vincristine (EMACO) was initiated from postoperative day 24 as follows: etoposide 100 mg/m2 of the body surface on days 1 and 2, methotrexate 300 mg/m2 of body surface on day 1, actinomycin D 0.5 mg on days 1 and 2, cyclophosphamide 600 mg/m2 of body surface on day 8, and vincristine 1 mg/m2 of body surface on day 8. After the first cycle of EMACO chemotherapy, the patient's serum β-hCG level decreased to 0.1 mIU/mL and CA-125 level decreased to 38.3 U/mL. However, the patient was unable to tolerate chemotherapy-induced emesis. Total parenteral nutrition was required for 3 weeks after first cycle of chemotherapy. Therefore, the regimen was altered to bleomycin, etoposide and cisplatin (BEP) chemotherapy: bleomycin 15 mg weekly for 3 doses, etoposide 100 mg/m2 of body surface on days 1 to 5, cisplatin 20 mg/m2 of body surface on days 1 to 5. After 2 cycles of BEP chemotherapy, moderate pulmonary insufficiency of obstructive type was identified by pulmonary function test. Therefore, the chemotherapy regimen was altered to etoposide, ifosfamide, and cisplatin (VIP) chemotherapy: etoposide 75 mg/m2 of body surface, ifosfamide 1.2 g/m2 of body surface, cisplatin 20 mg/m2 of body surface on days 1 to 5. No major side-effects occurred during the 3 cycles of VIP chemotherapy. The patient's CA-125 level returned to normal prior to the second cycle of BEP chemotherapy, and remained normal thereafter. Three months following chemotherapy the patient remained disease free.
Choriocarcinoma is a highly malignant tumor. Approximately 1 in 40,000 normal pregnancies and 1 in 40 hydatidiform moles proceed to gestational choriocarcinoma. Approximately 50% of choriocarcinoma cases are preceded by molar gestations, 25% by spontaneous abortions, 22.5% by normal pregnancy, and 2.5% by ectopic pregnancy [3]. Choriocarcinoma produces hCG, which is a useful tumor marker for screening, monitoring, and following up. Choriocarcinoma is characterized by a biphasic pattern of abnormal cytotrophoblast and syncytiotrophoblast with hyperplasia, anaplasia, hemorrhage, and necrosis [4]. Choriocarcinoma is categorized as either gestational or nongestational in origin. Unlike gestational choriocarcinoma, nongestational choriocarcinoma does not originate from molar or nonmolar pregnancy. Most commonly, nongestational choriocarcinoma arises from ovarian germ cell tumors, but can originate from other epithelial cancers such as lung, stomach, and bowel [5]. Because of the rarity of the disease, pathogenesis, clinical features, and treatment options are very limited. The pathological findings of nongestational choriocarcinoma are indistinguishable from gestational choriocarcinoma, and therefore diagnosis is difficult except in patients who are sexually immature, virgins, or postmenopausal. The diagnostic criteria, as described by Saito et al., include absence of disease in the uterine cavity, pathological confirmation of disease, and exclusion of molar pregnancy and of intrauterine pregnancy [2]. Our patient was using an intrauterine device and reported regular menstruation. All criteria were fulfilled in our case.
Several theories have been proposed regarding the pathogenesis of primary extraovarian choriocarcinoma. One theory is that choriocarcinoma develops from dedifferentiation of a poorly differentiated adenocarcinoma. Liu et al. [6] suggested that normal gastric mucosa could undergo trophoblastic metaplasia and neoplastic transformation. Another theory is that the tumor arises from germ cells that fail to migrate to the gonads [7]. In our case, the localization of the tumor was also in the germ cell migration pathway.
Choriocarcinoma is characterized by markedly elevated serum hCG. However, our patient was negative at the initial urine hCG test. The first explanation is that urine hCG tests might reveal false negative results. There are more than five variants of hCG in serum and urine samples in addition to regular hCG: hyperglycosylated hCG, nicked hCG missing the β-subunit, C-terminal peptide, free β-subunit, nicked free β-subunit, and multiple combinations of these variations. Currently, urine hCG tests use at least one antibody directed against the β-subunit of hCG. If a test kit detects only regular hCG, the result can be a false negative, because other hCG variants may predominate in patients with choriocarcinoma. Mehra et al. reported a case of choriocarcinoma with a negative serial urine β-hCG test that led to maternal mortality [8]. The second explanation is the "high-dose hook effect". There have been some reports describing false negative urine hCG in choriocarcinoma patients. This rare phenomenon occurs when the concentration of the antigen is very high, such that the antigen saturates both the solid migratory phase and fixed detection antibodies. According to these reports, β-hCG levels were greater than 1,000,000 mIU/mL. About 1:10 to 1:1000 dilutions of urine samples are helpful for overcoming the hook effect [9,10].
In our patient, choriocarcinoma was also present in the paracervical area. There are two possible explanations for the pathogenesis of this tumor, other than the possibility that it was a metastastatic tumor arising from primary retroperitoneal choriocarcinoma. First, choriocarcinoma might have arisen at the paracervical region and metastasized to the retroperitoneum. Thomakos et al. [1] reported a case of a 39-year-old woman with retroperitoneal metastatic gestational trophoblastic neoplasia. Second, there may have been a preceding ectopic pregnancy that led to choriocarcinoma.
The clinical manifestation of choriocarcinoma is variable. About one-third of patients suffer from symptoms of distant metastasis [11]. Menorrhagia is the most common clinical sign. In patients with pulmonary metastasis, chronic cough, dyspnea, or hemoptysis can occur, but usually remains asymptomatic until metastatic lesions are identified on routine chest radiography [12]. In our case, the clinical symptoms were lower abdominal pain, nausea, and fever related to hemorrhagic necrosis of the tumor. Thus, clinicians should consider the possibility of choriocarcinoma in any patient of childbearing age with a metastatic malignant lesion.
Treatment of choriocarcinoma is primarily based on a chemotherapeutic approach. In patients with high-risk metastatic disease, multi-agent chemotherapy is the treatment of choice. Adjuvant surgery or radiotherapy can also be considered for these patients. The EMACO protocol or variations are currently the treatment of choice for patients with high risk metastatic disease. The low toxicity of EMACO chemotherapy allows patients to adhere to treatment schedules, and it yields high response rates and overall survival [13]. The complete remission rate of patients with stage IV choriocarcinoma is 80%, with a relapse rate of less than 10% [14,15]. Chemotherapy with etoposide and platinum agents, with or without bleomycin or ifosfamide, is used for patients with resistant or relapsed disease [15]. Bleomycin, etoposide, and cisplatin (BEP) chemotherapy is the most commonly used second-line regimen, and cisplatin, vinblastine, and bleomycin chemotherapy is also widely used. Lurain and Nejad explored the efficacy of the BEP regimen as a secondary chemotherapy, which yielded 74% (14/19) in complete responses rate and 58% (11/19) in overall survival rate [13].
In summary, we report a rare case of retroperitoneal nongestational choriocarcinoma with negative urine β-hCG. The diagnosis of extrauterine choriocarcinoma is difficult due to its unusual clinical manifestations, but immunohistopathology is the mainstay of diagnosis. As hCG tests may show true or false negative results, they cannot rule out choriocarcinoma completely.
Figures and Tables
 | Fig. 1(A) Transabdominal ultrasonographic findings of the mass in left adnexal region. The mass is encircling left common and external iliac artery. (B) Magnetic resonance imaging shows heterogenous enhancing mass (arrow) in left retroperitoneum which is encircling left iliac vessel. (C) Positron emission tomography demonstrates suspicious malignant mass in left iliac region, recto-uterine pouch, and left side of the uterus. |
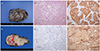 | Fig. 2(A) Gross photography. (B) The cut section of the tumor shows a hemorrhage and some necrotic portion with left external iliac artery (arrow) inside. (C) Microscopic findings of the tumor. Multinucleated syncytiotrophoblast cells are growing over nest of cytotrophoblasts in a pelxiform pattern (H&E, ×100). (D) Immunoreactive for p63 immunostaining (p63, ×100). (E) The tumor cells are immunoreacitve for cytokeratin 7 (CK7, ×100). (F) The syncytiotrophoblast cells express β-human chorionic gonadotropin (β-hCG, ×100). |
References
1. Thomakos N, Rodolakis A, Belitsos P, Zagouri F, Chatzinikolaou I, Dimopoulos AM, et al. Gestational trophoblastic neoplasia with retroperitoneal metastases: a fatal complication. World J Surg Oncol. 2010; 8:114.
2. Saito M, Azuma T, Nakamura K. On ectopic choriocarcinoma. World Obstet Gynecol. 1965; 17:459–484.
3. Bentley RC. Pathology of gestational trophoblastic disease. Clin Obstet Gynecol. 2003; 46:513–522.
4. Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010; 376:717–729.
5. Oladipo A, Mathew J, Oriolowo A, Lindsay I, Fisher R, Seckl M, et al. Nongestational choriocarcinoma arising from a primary ovarian tumour. BJOG. 2007; 114:1298–1300.
6. Liu Z, Mira JL, Cruz-Caudillo JC. Primary gastric choriocarcinoma: a case report and review of the literature. Arch Pathol Lab Med. 2001; 125:1601–1604.
7. Reid JD. Neoplastic structure and function as expressions of genetic information systems. N Z Med J. 1970; 71:303–304.
8. Mehra R, Huria A, Gupta P, Mohan H. Choriocarcinoma with negative urinary and serum beta human chorionic gonadotropin (betaHCG): a case report. Indian J Med Sci. 2005; 59:538–541.
9. Olaniyan OB, Momoh JA. Negative urine hCG in choriocarcinoma. Int J Gynaecol Obstet. 2007; 98:59–60.
10. Hunter CL, Ladde J. Molar pregnancy with false negative β-hCG urine in the emergency department. West J Emerg Med. 2011; 12:213–215.
11. Ngan S, Seckl MJ. Gestational trophoblastic neoplasia management: an update. Curr Opin Oncol. 2007; 19:486–491.
12. Kumar J, Ilancheran A, Ratnam SS. Pulmonary metastases in gestational trophoblastic disease: a review of 97 cases. Br J Obstet Gynaecol. 1988; 95:70–74.
13. Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011; 204:11–18.
14. Berkowitz RS, Goldstein DP. Current advances in the management of gestational trophoblastic disease. Gynecol Oncol. 2013; 128:3–5.
15. Lurain JR, Nejad B. Secondary chemotherapy for high-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2005; 97:618–623.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download